Characterization of a whole blood assay for quantifying myeloid-derived suppressor cells
- PMID: 31462270
- PMCID: PMC6714080
- DOI: 10.1186/s40425-019-0674-1
Characterization of a whole blood assay for quantifying myeloid-derived suppressor cells
Abstract
Background: Myeloid-derived suppressor cells (MDSC) have been found to play an important role in limiting immune responses in cancer. Higher circulating MDSC levels have been associated with greater tumor burden, poorer response to immunotherapy, and poorer survival. Optimal measurement of MDSC levels could provide clinicians with a useful prognostic and/or management tool.
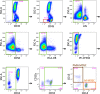
Methods: A whole blood (WB) nine color, 11 parameter flow cytometric assay was designed, utilizing fluorescently-labeled antibodies against CD45, CD3, CD19, CD20, CD56, CD16, HLA-DR, CD33, CD11b, CD14 and CD15, and BD Trucount beads for quantitation. Total MDSC were defined as CD45 + CD3-CD19-CD20-CD56-CD16-HLA-DR-CD33 + CD11b + cells, while the monocytic (M-MDSC) and polymorphonuclear subsets were defined as CD14+ or CD15+, respectively.
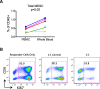
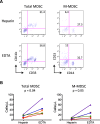
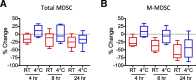
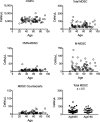
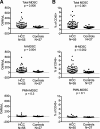
Results: A novel gating strategy was devised to eliminate granulocytes and improve consistency in gating. Several pre-analytical variables were found to significantly affect MDSC quantitation, including collection tube type and time elapsed between blood collection and testing. Total and M-MDSC levels were a mean of 63% and 73% greater, respectively, with K2EDTA compared to Na+heparin collection tubes (N = 5). In addition, time elapsed at room temperature prior to cell labeling affected MDSC quantitation; by 24 h after blood collection, total and M-MDSC levels were a mean of 26% and 57% lower compared to testing as soon as possible after collection (N = 6). Refrigeration of samples at 4 °C ameliorated time-dependent effects at both 4 and 8 h, but not 24 h after blood collection. To establish normal ranges for this assay, MDSC levels were quantified in 67 healthy subjects (30 male, 37 female) ages 20-93. No significant differences in total or M-MDSC levels were detected for ages ≤60 compared to > 60 (p = 0.5 and p = 0.8, respectively). Finally, assay results demonstrated significantly higher MDSC levels among patients with hepatocellular carcinoma (N = 55) compared to age-matched healthy controls (N = 27) for total and M-MDSC (p = 0.006 and 0.004, respectively).
Conclusions: MDSC are a heterogenous group of cells, and their quantitation in WB can be affected by a number of pre-analytical variables. Consideration of these factors, and measurement using a material type that has not been manipulated, such as whole blood, is likely to yield the most accurate results.
Keywords: Flow cytometry; Immunotherapy; Liver cancer; Myeloid-derived suppressor cells; Whole blood.
Conflict of interest statement
The authors declare they have no competing interests.
Figures
References
-
- Sade-Feldman M, Kanterman J, Klieger Y, Ish-Shalom E, Olga M, Saragovi A, et al. Clinical significance of circulating CD33+CD11b+HLA-DR- myeloid cells in patients with stage IV melanoma treated with Ipilimumab. Clin Cancer Res. 2016;22(23):5661–5672. - PubMed
-
- Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin Cancer Res. 2014;20(6):1601–1609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
