Inhaled corticosteroid suppression of cathelicidin drives dysbiosis and bacterial infection in chronic obstructive pulmonary disease
- PMID: 31462509
- PMCID: PMC7237237
- DOI: 10.1126/scitranslmed.aav3879
Inhaled corticosteroid suppression of cathelicidin drives dysbiosis and bacterial infection in chronic obstructive pulmonary disease
Abstract
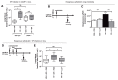
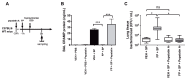
Bacterial infection commonly complicates inflammatory airway diseases such as chronic obstructive pulmonary disease (COPD). The mechanisms of increased infection susceptibility and how use of the commonly prescribed therapy inhaled corticosteroids (ICS) accentuates pneumonia risk in COPD are poorly understood. Here, using analysis of samples from patients with COPD, we show that ICS use is associated with lung microbiota disruption leading to proliferation of streptococcal genera, an effect that could be recapitulated in ICS-treated mice. To study mechanisms underlying this effect, we used cellular and mouse models of streptococcal expansion with Streptococcus pneumoniae, an important pathogen in COPD, to demonstrate that ICS impairs pulmonary clearance of bacteria through suppression of the antimicrobial peptide cathelicidin. ICS impairment of pulmonary immunity was dependent on suppression of cathelicidin because ICS had no effect on bacterial loads in mice lacking cathelicidin (Camp -/-) and exogenous cathelicidin prevented ICS-mediated expansion of streptococci within the microbiota and improved bacterial clearance. Suppression of pulmonary immunity by ICS was mediated by augmentation of the protease cathepsin D. Collectively, these data suggest a central role for cathepsin D/cathelicidin in the suppression of antibacterial host defense by ICS in COPD. Therapeutic restoration of cathelicidin to boost antibacterial immunity and beneficially modulate the lung microbiota might be an effective strategy in COPD.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures




References
-
- Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. The New England journal of medicine. 2002 Aug 15;347:465. - PubMed
-
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 1998 May;157:1418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

