Phagocytosis checkpoints as new targets for cancer immunotherapy
- PMID: 31462760
- PMCID: PMC7002027
- DOI: 10.1038/s41568-019-0183-z
Phagocytosis checkpoints as new targets for cancer immunotherapy
Abstract
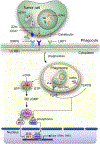
Cancer immunotherapies targeting adaptive immune checkpoints have substantially improved patient outcomes across multiple metastatic and treatment-refractory cancer types. However, emerging studies have demonstrated that innate immune checkpoints, which interfere with the detection and clearance of malignant cells through phagocytosis and suppress innate immune sensing, also have a key role in tumour-mediated immune escape and might, therefore, be potential targets for cancer immunotherapy. Indeed, preclinical studies and early clinical data have established the promise of targeting phagocytosis checkpoints, such as the CD47-signal-regulatory protein α (SIRPα) axis, either alone or in combination with other cancer therapies. In this Review, we highlight the current understanding of how cancer cells evade the immune system by disrupting phagocytic clearance and the effect of phagocytosis checkpoint blockade on induction of antitumour immune responses. Given the role of innate immune cells in priming adaptive immune responses, an improved understanding of the tumour-intrinsic processes that inhibit essential immune surveillance processes, such as phagocytosis and innate immune sensing, could pave the way for the development of highly effective combination immunotherapy strategies that modulate both innate and adaptive antitumour immune responses.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

