Hallmarks of Aging in the Liver
- PMID: 31462971
- PMCID: PMC6709368
- DOI: 10.1016/j.csbj.2019.07.021
Hallmarks of Aging in the Liver
Abstract
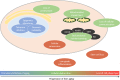
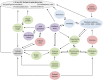
While the liver demonstrates remarkable resilience during aging, there is growing evidence that it undergoes all the cellular hallmarks of aging, which increases the risk of liver and systemic disease. The aging process in the liver is driven by alterations of the genome and epigenome that contribute to dysregulation of mitochondrial function and nutrient sensing pathways, leading to cellular senescence and low-grade inflammation. These changes promote multiple phenotypic changes in all liver cells (hepatocytes, liver sinusoidal endothelial, hepatic stellate and Küpffer cells) and impairment of hepatic function. In particular, age-related changes in the liver sinusoidal endothelial cells are a significant but under-recognized risk factor for the development of age-related cardiometabolic disease.
Keywords: AMPK, 5′ adenosine monophosphate-activated protein kinase; CR, caloric restriction; Endothelial; FOXO, forkhead box O; Genetic; HSC, hepatic stellate cell; Hepatocyte; IGF-1, insulin like growth factor 1; IL-6, interleukin 6; IL-8, interleukin 8; KC, Küpffer cell; LSEC, liver sinusoidal endothelial cell; Mitochondrial dysfunction; NAD, nicotinamide adenine dinucleotide; NAFLD, non-alcoholic fatty liver disease; NO, nitric oxide; Nutrient sensing pathways; PDGF, platelet derived growth factor; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-α; ROS, reactive oxygen species; SIRT1, sirtuin 1; Senescence; TNFα, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor; mTOR, mammalian target of rapamycin; miR, microRNA; αSMA, alpha smooth muscle actin.
Conflict of interest statement
None.
Figures


References
-
- Niccoli T., Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22(17) [R741-R52] - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
