Lead (Pb) exposure induces dopaminergic neurotoxicity in Caenorhabditis elegans: Involvement of the dopamine transporter
- PMID: 31463204
- PMCID: PMC6709386
- DOI: 10.1016/j.toxrep.2019.08.001
Lead (Pb) exposure induces dopaminergic neurotoxicity in Caenorhabditis elegans: Involvement of the dopamine transporter
Abstract
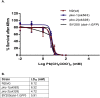
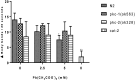
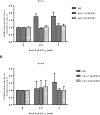
Lead (Pb) is an environmental neurotoxicant, and has been implicated in several neurological disorders of dopaminergic dysfunction; however, the molecular mechanism of its toxicity has yet to be fully understood. This study investigated the effect of Pb exposure on dopaminergic neurodegeneration and function, as well as expression level of several dopaminergic signaling genes in wild type (N2) and protein kinase C (pkc) mutant Caenorhabditis elegans. Both N2 and pkc mutant worms were exposed to Pb2+ for 1 h. Thereafter, dopaminergic (DAergic) neurodegeneration, behavior and gene expression levels were assessed. The results revealed that Pb2+ treatment affects dopaminergic cell morphology and structure in worms expressing green fluorescent protein (GFP) under a DAergic cell specific promoter. Also, there was a significant impairment in dopaminergic neuronal function as tested by basal slowing response (BSR) in wild-type, N2 worms, but no effect was observed in pkc mutant worms. Furthermore, Pb2+ exposure increased dat-1 gene expression level when compared with N2 worms, but no alteration was observed in the pkc mutant strains. LC-MS analysis revealed a significant decrease in dopamine content in worms treated with Pb2+ when compared with controls. In summary, our results revealed that Pb2+ exposure induced dopaminergic dysfunction in C. elegans by altering dat-1 gene levels, but pkc mutants showed significant resistance to Pb2+ toxicity. We conclude that PKC activation is directly involved in the neurotoxicity of Pb.
Keywords: Dopaminergic neuron; Neurotoxicant; PKC; Pb; dat-1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

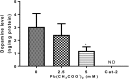


References
-
- Iversen S.D., Iversen L.L. Dopamine: 50 years in perspective. Trends Neurosci. 2007;30:188–193. - PubMed
-
- Kristensen A.S., Andersen J., Jorgensen T.N., Sorensen L., Eriksen J., Loland C.J., Stromgaard K., Gether U. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol. Rev. 2011;63:585–640. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

