7-Ketocholesterol and cholestane-triol increase expression of SMO and LXRα signaling pathways in a human breast cancer cell line
- PMID: 31463370
- PMCID: PMC6709374
- DOI: 10.1016/j.bbrep.2018.12.008
7-Ketocholesterol and cholestane-triol increase expression of SMO and LXRα signaling pathways in a human breast cancer cell line
Abstract
Oxysterols are 27-carbon oxidation products of cholesterol metabolism. Oxysterols possess several biological actions, including the promotion of cell death. Here, we examined the ability of 7-ketocholesterol (7-KC), cholestane-3β-5α-6β-triol (triol), and a mixture of 5α-cholestane-3β,6β-diol and 5α-cholestane-3β,6α-diol (diol) to promote cell death in a human breast cancer cell line (MDA-MB-231). We determined cell viability, after 24-h incubation with oxysterols. These oxysterols promoted apoptosis. At least part of the observed effects promoted by 7-KC and triol arose from an increase in the expression of the sonic hedgehog pathway mediator, smoothened. However, this increased expression was apparently independent of sonic hedgehog expression, which did not change. Moreover, these oxysterols led to increased expression of LXRα, which is involved in cellular cholesterol efflux, and the ATP-binding cassette transporters, ABCA1 and ABCG1. Diols did not affect these pathways. These results suggested that the sonic hedgehog and LXRα pathways might be involved in the apoptotic process promoted by 7-KC and triol.
Keywords: ABC transporters; Apoptosis; Cancer; LXRα; Oxysterol; Sonic hedgehog.
Figures

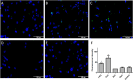

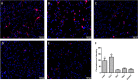
References
-
- Howe V., Sharpe L.J., Alexopoulos S.J. Cholesterol homeostasis: how do cells sense sterol excess? Chem. Phys. Lipids. 2016;199:170–178. - PubMed
-
- Jakobsson T., Treuter E., Gustafsson J.A. Liver X receptor biology and pharmacology: new pathways, challenges and opportunities. Trends Pharmacol. Sci. 2012;33:394–404. - PubMed
-
- Carvalho J.F., Silva M.M., Moreira J.N. Selective cytotoxicity of oxysterols through structural modulation on rings A and B. Synthesis, in vitro evaluation, and SAR. J. Med. Chem. 2011;54:6375–6393. - PubMed
-
- Kulig W., Cwiklik L., Jurkiewicz P. Cholesterol oxidation products and their biological importance. Chem. Phys. Lipids. 2016;199:144–160. - PubMed
-
- Zerbinati C., Iuliano L. Cholesterol and related sterols autoxidation. Free Radic. Biol. Med. 2017;111:151–155. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

