Agreement of amyloid PET and CSF biomarkers for Alzheimer's disease on Lumipulse
- PMID: 31464088
- PMCID: PMC6764494
- DOI: 10.1002/acn3.50873
Agreement of amyloid PET and CSF biomarkers for Alzheimer's disease on Lumipulse
Abstract
Objective: To determine the cutoffs that optimized the agreement between 18 F-Florbetapir positron emission tomography (PET) and Aβ1-42, Aβ1-40, tTau, pTau and their ratios measured in cerebrospinal fluid (CSF) on the LUMIPULSE G600II instrument, we quantified the levels of these four biomarkers in 94 CSF samples from participants of the Sant Pau Initiative on Neurodegeneration (SPIN cohort) using the Lumipulse G System with available 18 F-Florbetapir imaging.
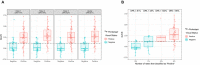
Methods: Participants had mild cognitive impairment (n = 35), AD dementia (n = 12), other dementias or neurodegenerative diseases (n = 41), or were cognitively normal controls (n = 6). Levels of Aβ1-42 were standardized to certified reference material. Amyloid scans were assessed visually and through automated quantification. We determined the cutoffs of CSF biomarkers that optimized their agreement with 18 F-Florbetapir PET and evaluated concordance between markers of the amyloid category.
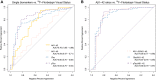
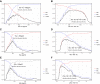
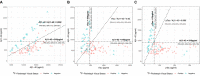
Results: Aβ1-42, tTau and pTau (but not Aβ1-40) and the ratios with Aβ1-42 had good diagnostic agreement with 18 F-Florbetapir PET. As a marker of amyloid pathology, the Aβ1-42/Aβ1-40 ratio had higher agreement and better correlation with amyloid PET than Aβ1-42 alone.
Interpretation: CSF biomarkers measured with the Lumipulse G System show good agreement with amyloid imaging in a clinical setting with heterogeneous presentations of neurological disorders. Combination of Aβ1-42 with Aβ1-40 increases the agreement between markers of amyloid pathology.
© 2019 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association.
Conflict of interest statement
D.A. participated in advisory boards from Fujirebio‐Europe and received speaker honoraria from Fujirebio‐Europe, Nutricia and from Krka Farmacéutica S.L. R.B. participated in advisory boards from Lilly and Nutricia, and he received speaker honoraria and travel funding from Novartis and Nutricia. A.L. participated in advisory boards from Fujirebio‐Europe, Nutricia, Biogen, and received speaker honoraria from Lilly. N.LB. and E.H. are employed by Fujirebio Europe. N.V. A.N. and V.O. are employed by Fujirebio Iberia, S.L.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- CIBERNED/International
- 20141210/Fundació la Marató de TV3/International
- 20142610/Fundació la Marató de TV3/International
- 20161431/Fundació la Marató de TV3/International
- PI13/01532/Instituto de Salud Carlos III/International
- PI14/01126/Instituto de Salud Carlos III/International
- PI14/01561/Instituto de Salud Carlos III/International
- PI16/01825/Instituto de Salud Carlos III/International
- PI17/01019/Instituto de Salud Carlos III/International
- PI17/01895/Instituto de Salud Carlos III/International
- PI18/00435/Instituto de Salud Carlos III/International
- 2017-SGR-547/Agència de Gestió d'Ajuts Universitaris i de Recerca/International
- SLT006/17/119/Agència de Gestió d'Ajuts Universitaris i de Recerca/International
- SLT006/17/125/Agència de Gestió d'Ajuts Universitaris i de Recerca/International
- SLT006/17/95/Agència de Gestió d'Ajuts Universitaris i de Recerca/International
- la Caixa Foundation/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

