Bacterial ribonucleases and their roles in RNA metabolism
- PMID: 31464530
- PMCID: PMC6776250
- DOI: 10.1080/10409238.2019.1651816
Bacterial ribonucleases and their roles in RNA metabolism
Abstract
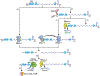
Ribonucleases (RNases) are mediators in most reactions of RNA metabolism. In recent years, there has been a surge of new information about RNases and the roles they play in cell physiology. In this review, a detailed description of bacterial RNases is presented, focusing primarily on those from Escherichia coli and Bacillus subtilis, the model Gram-negative and Gram-positive organisms, from which most of our current knowledge has been derived. Information from other organisms is also included, where relevant. In an extensive catalog of the known bacterial RNases, their structure, mechanism of action, physiological roles, genetics, and possible regulation are described. The RNase complement of E. coli and B. subtilis is compared, emphasizing the similarities, but especially the differences, between the two. Included are figures showing the three major RNA metabolic pathways in E. coli and B. subtilis and highlighting specific steps in each of the pathways catalyzed by the different RNases. This compilation of the currently available knowledge about bacterial RNases will be a useful tool for workers in the RNA field and for others interested in learning about this area.
Keywords: RNases; RNA processing; RNase function; RNase mechanism; RNase regulation; RNase structure; mRNA decay.
Conflict of interest statement
Disclosure statement
No potential conflict of interest was reported by the authors.
Figures
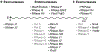



References
-
- Aguilera A, Garcia-Muse T. 2012. R loops: from transcription byproducts to threats to genome stability. Mol Cell. 46(2):115–124. - PubMed
-
- Alifano P, Rivellini F, Piscitelli C, Arraiano CM, Bruni CB, Carlomagno MS. 1994. Ribonuclease E provides substrates for ribonuclease P-dependent processing of a polycistronic mRNA. Genes Dev. 8(24):3021–3031. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
