Darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-experienced, virologically suppressed patients with HIV-1: subgroup analyses of the phase 3 EMERALD study
- PMID: 31464642
- PMCID: PMC6716878
- DOI: 10.1186/s12981-019-0235-1
Darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-experienced, virologically suppressed patients with HIV-1: subgroup analyses of the phase 3 EMERALD study
Abstract
Background: Darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) 800/150/200/10 mg is a once-daily, single-tablet regimen for treatment of HIV-1 infection. The efficacy/safety of switching to D/C/F/TAF versus continuing boosted protease inhibitor (bPI) + emtricitabine/tenofovir disoproxil fumarate (control) were demonstrated in a phase 3, randomized study (EMERALD) of treatment-experienced, virologically suppressed adults through week 48. The objective of this analysis was to evaluate EMERALD outcomes across subgroups of patients based on demographic characteristics, prior treatment experience, and baseline antiretroviral regimen.
Methods: EMERALD patients were virologically suppressed (viral load [VL] < 50 copies/mL for ≥ 2 months at screening). Prior non-darunavir virologic failure (VF) was allowed. Primary endpoint was proportion of patients with virologic rebound (confirmed VL ≥ 50 copies/mL) cumulative through week 48. Virologic response was VL < 50 copies/mL (FDA snapshot). Safety was assessed by adverse events, renal proteinuria markers, and bone mineral density. Outcomes were examined for prespecified subgroups by age (≤/> 50 years), gender, race (black/non-black), prior number of antiretrovirals used (4/5/6/7/> 7), prior VF (0/≥ 1), baseline bPI (darunavir/atazanavir or lopinavir), and baseline boosting agent (ritonavir/cobicistat).
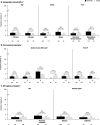
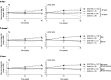
Results: Among 1141 patients in the D/C/F/TAF (n = 763) and control (n = 378) arms, virologic rebound rates (2.5% and 2.1%, respectively) were similar, and this was consistent across all subgroups. Virologic response rates ranged from 91 to 97% (D/C/F/TAF) and 89 to 99% (control) across all subgroups, with differences between treatment arms of 0 and 6%. Adverse event rates were low in both arms and across subgroups. Improvements in renal and bone parameters were observed with D/C/F/TAF across demographic subgroups.
Conclusions: For treatment-experienced, virologically suppressed patients, switching to D/C/F/TAF was highly effective and safe, regardless of demographic characteristics, prior treatment experience, or pre-switch bPI. Trial registration ClinicalTrials.gov Identifier: NCT02269917. Registered 21 October 2014. https://clinicaltrials.gov/ct2/show/NCT02269917.
Keywords: Antiretroviral; Darunavir; HIV-1; Protease inhibitor; Single-tablet regimen; Switch study.
Conflict of interest statement
GDH has received research or grant support from Gilead, ViiV, Janssen, Proteus, and the US National Institutes of Health; he has also served as a consultant to Janssen, Gilead, and ViiV. JJE received research grants from Janssen, Gilead, and ViiV; and has served as a consultant to Bristol-Myers Squibb, Merck, Janssen, Gilead, and ViiV. P-MG has received grant/research support from Merck; served on advisory committees or review panels for Merck, Roche, ViiV, Gilead, and Janssen; and served as a speaker/teacher for Merck and Tibotec. CO has received speaker honoraria or consulting fees for attending speakers bureaus or advisory boards for, and has received research grants from, Janssen, Merck, ViiV, and Gilead. J-MM has received honoraria for participation in advisory boards for Gilead and Merck; and has received a research grant from Merck. EDJ has participated in a speaker bureau for Gilead, and advisory boards for Gilead, Janssen, and Theratechnologies. RP is a former contractor for Janssen. DL, EVL, EL, REN, KB, and EYW are full-time employees of Janssen.
Figures


References
-
- Orkin C, DeJesus E, Khanlou H, Stoehr A, Supparatpinyo K, Lathouwers E, et al. Final 192-week efficacy and safety of once-daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naive patients in the ARTEMIS trial. HIV Med. 2013;14(1):49–59. doi: 10.1111/j.1468-1293.2012.01060.x. - DOI - PubMed
-
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 25 Oct 2018.
-
- European AIDS Clinical Society. EACS guidelines version 9.1, 2018.
-
- Orkin C, Molina J-M, Negredo E, Arribas JR, Gathe J, Eron JJ, et al. Efficacy and safety of switching from boosted protease inhibitors plus emtricitabine and tenofovir disoproxil fumarate regimens to single-tablet darunavir, cobicistat, emtricitabine, and tenofovir alafenamide at 48 weeks in adults with virologically suppressed HIV-1 (EMERALD): a phase 3, randomised, non-inferiority trial. Lancet HIV. 2018;5(1):e23–e34. doi: 10.1016/S2352-3018(17)30179-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

