Epidemiology and Outcome of Critically Ill Pediatric Cancer and Hematopoietic Stem Cell Transplant Patients Requiring Continuous Renal Replacement Therapy: A Retrospective Nationwide Cohort Study
- PMID: 31464768
- PMCID: PMC6798750
- DOI: 10.1097/CCM.0000000000003973
Epidemiology and Outcome of Critically Ill Pediatric Cancer and Hematopoietic Stem Cell Transplant Patients Requiring Continuous Renal Replacement Therapy: A Retrospective Nationwide Cohort Study
Abstract
Objective: Acute kidney injury requiring continuous renal replacement therapy is a serious treatment-related complication in pediatric cancer and hematopoietic stem cell transplant patients. The purpose of this study was to assess epidemiology and outcome of these patients requiring continuous renal replacement therapy in the PICU.
Design: A nationwide, multicenter, retrospective, observational study.
Setting: Eight PICUs of a tertiary care hospitals in the Netherlands.
Patients: Pediatric cancer and hematopoietic stem cell transplant patients (cancer and noncancer) who received continuous renal replacement therapy from January 2006 to July 2017 in the Netherlands.
Interventions: None.
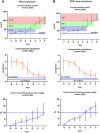
Measurement and main results: Of 1,927 PICU admissions of pediatric cancer and hematopoietic stem cell transplant patients, 68 of 70 evaluable patients who received continuous renal replacement therapy were included. Raw PICU mortality was 11.2% (216/1,972 admissions). PICU mortality of patients requiring continuous renal replacement therapy was 54.4% (37/68 patients). Fluid overload (odds ratio, 1.08; 95% CI, 1.01-1.17) and need for inotropic support (odds ratio, 6.53; 95% CI, 1.86-23.08) at the start of continuous renal replacement therapy were associated with PICU mortality. Serum creatinine levels increased above 150% of baseline 3 days before the start of continuous renal replacement therapy. Urine production did not reach the critical limit of oliguria. In contrast, body weight (fluid overload) increased already 5 days prior to continuous renal replacement therapy initiation.
Conclusions: PICU mortality of pediatric cancer and hematopoietic stem cell transplant patients requiring continuous renal replacement therapy is sadly high. Fluid overload at the initiation of continuous renal replacement therapy is the most important and earliest predictor of PICU mortality. Our results suggest that the most commonly used criteria of acute kidney injury, that is, serum creatinine and urine production, are not useful as a trigger to initiate continuous renal replacement therapy. This highlights the urgent need for prospective studies to generate recommendations for effective therapeutic interventions at an early phase in this specific patient population.
Conflict of interest statement
The authors have disclosed that they do not have any potential conflicts of interest.
Figures

References
-
- Bleyer WA. The U.S. pediatric cancer clinical trials programmes: International implications and the way forward. Eur J Cancer 1997; 33:1439–1447 - PubMed
-
- Petridou ET, Dimitrova N, Eser S, et al. Childhood leukemia and lymphoma: Time trends and factors affecting survival in five southern and eastern European cancer registries. Cancer Causes Control 2013; 24:1111–1118 - PubMed
-
- Pritchard-Jones K, Kaatsch P, Steliarova-Foucher E, et al. Cancer in children and adolescents in Europe: Developments over 20 years and future challenges. Eur J Cancer 2006; 42:2183–2190 - PubMed
-
- Dalton HJ, Slonim AD, Pollack MM. MultiCenter outcome of pediatric oncology patients requiring intensive care. Pediatr Hematol Oncol 2003; 20:643–649 - PubMed
-
- Kress JP, Christenson J, Pohlman AS, et al. Outcomes of critically ill cancer patients in a university hospital setting. Am J Respir Crit Care Med 1999; 160:1957–1961 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

