Clinical Effectiveness of Intravitreal Therapy With Ranibizumab vs Aflibercept vs Bevacizumab for Macular Edema Secondary to Central Retinal Vein Occlusion: A Randomized Clinical Trial
- PMID: 31465100
- PMCID: PMC6865295
- DOI: 10.1001/jamaophthalmol.2019.3305
Clinical Effectiveness of Intravitreal Therapy With Ranibizumab vs Aflibercept vs Bevacizumab for Macular Edema Secondary to Central Retinal Vein Occlusion: A Randomized Clinical Trial
Abstract
Importance: The comparative clinical effectiveness of ranibizumab, aflibercept, and bevacizumab for the management of macular edema due to central retinal vein occlusion (CRVO) is unclear.
Objective: To determine whether intravitreal aflibercept or bevacizumab compared with ranibizumab results in a noninferior mean change in vision at 100 weeks for eyes with CRVO-related macular edema.
Design, setting, and participants: This prospective, 3-arm, double-masked, randomized noninferiority trial (Lucentis, Eylea, Avastin in Vein Occlusion [LEAVO] Study) took place from December 12, 2014, through December 16, 2016, at 44 UK National Health Service ophthalmology departments. Inclusion criteria included age 18 years or older, visual impairment due to CRVO-related macular edema of less than 12 months with best-corrected visual acuity (BCVA) Early Treatment Diabetic Retinopathy Study letter score (approximate Snellen equivalent) in the study eye between 19 (20/400) and 78 (20/32), and spectral domain optical coherence tomography imaging central subfield thickness of 320 μm or greater. Data were analyzed from March 4, 2019, to April 26, 2019.
Interventions: Participants were randomized (1:1:1) to receive repeated intravitreal injections of ranibizumab (0.5 mg/0.05 mL) (n = 155), aflibercept (2.0 mg/0.05 mL) (n = 154), or bevacizumab (1.25 mg/0.05 mL) (n = 154) for 100 weeks.
Main outcomes and measures: Adjusted mean change in BCVA in the study eye at 100 weeks wherein noninferiority was concluded if the lower bounds of the 95% CI of both the intention-to-treat and the per protocol analyses were above -5 letters.
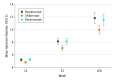
Results: Of 463 participants, 265 (57.2%) were male, with a mean (SD) age of 69.1 (13.0) years. The mean (SD) gain in BCVA letter score was 12.5 (21.1) for ranibizumab, 15.1 (18.7) for aflibercept, and 9.8 (21.4) for bevacizumab at 100 weeks. Aflibercept was noninferior to ranibizumab (intention-to-treat-adjusted mean BCVA difference, 2.23 letters; 95% CI, -2.17 to 6.63 letters; P < .001). Bevacizumab was not noninferior to ranibizumab (intention-to-treat-adjusted mean BCVA difference, -1.73 letters; 95% CI, -6.12 to 2.67 letters; P = .07). The per protocol analysis conclusions were similar. Fewer mean injections were given in the aflibercept group (10.0) than in the ranibizumab (11.8) group (mean difference at 100 weeks, -1.9; 95% CI, -2.9 to -0.8).
Conclusions and relevance: Mean changes in vision after treatment of macular edema due to CRVO were no worse using aflibercept compared with ranibizumab. Mean changes in vision using bevacizumab compared with ranibizumab were inconclusive regarding vision outcomes (ie, the change in visual acuity from baseline, on average, may be worse or may not be worse when using bevacizumab compared with ranibizumab).
Trial registration: ISRCTN13623634.
Conflict of interest statement
Figures

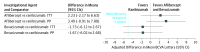
References
-
- Rogers S, McIntosh RL, Cheung N, et al. ; International Eye Disease Consortium . The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117(2):313-319.e1. doi:10.1016/j.ophtha.2009.07.017 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

