Anatomically accurate model of EMG during index finger flexion and abduction derived from diffusion tensor imaging
- PMID: 31465437
- PMCID: PMC6738720
- DOI: 10.1371/journal.pcbi.1007267
Anatomically accurate model of EMG during index finger flexion and abduction derived from diffusion tensor imaging
Abstract
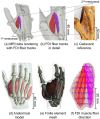
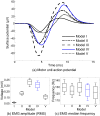
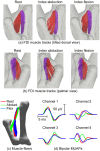
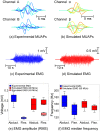
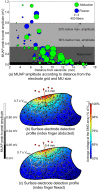
This study presents a modelling framework in which information on muscle fiber direction and orientation during contraction is derived from diffusion tensor imaging (DTI) and incorporated in a computational model of the surface electromyographic (EMG) signal. The proposed model makes use of the principle of reciprocity to simultaneously calculate the electric potentials produced at the recording electrode by charges distributed along an arbitrary number of muscle fibers within the muscle, allowing for a computationally efficient evaluation of extracellular motor unit action potentials. The approach is applied to the complex architecture of the first dorsal interosseous (FDI) muscle of the hand to simulate EMG during index finger flexion and abduction. Using diffusion tensor imaging methods, the results show how muscle fiber orientation and curvature in this intrinsic hand muscle change during flexion and abduction. Incorporation of anatomically accurate muscle architecture and other hand tissue morphologies enables the model to capture variations in extracellular action potential waveform shape across the motor unit population and to predict experimentally observed differences in EMG signal features when switching from index finger abduction to flexion. The simulation results illustrate how structural and electrical properties of the tissues comprising the volume conductor, in combination with fiber direction and curvature, shape the detected action potentials. Using the model, the relative contribution of motor units of different sizes located throughout the muscle under both conditions is examined, yielding a prediction of the detection profile of the surface EMG electrode array over the muscle cross-section.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

