Real-world cost-effectiveness of pan-genotypic Sofosbuvir-Velpatasvir combination versus genotype dependent directly acting anti-viral drugs for treatment of hepatitis C patients in the universal coverage scheme of Punjab state in India
- PMID: 31465503
- PMCID: PMC6715223
- DOI: 10.1371/journal.pone.0221769
Real-world cost-effectiveness of pan-genotypic Sofosbuvir-Velpatasvir combination versus genotype dependent directly acting anti-viral drugs for treatment of hepatitis C patients in the universal coverage scheme of Punjab state in India
Abstract
Background: We undertook this study to assess the incremental cost per quality adjusted life year (QALY) gained with the use of pan-genotypic sofosbuvir (SOF) + velpatasvir (VEL) for HCV patients, as compared to the current treatment regimen under the universal free treatment scheme in Punjab state.
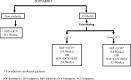
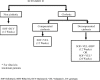
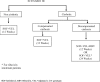
Methodology: A Markov model depicting natural history of HCV was developed to simulate the progression of disease. Three scenarios were compared: I (Current Regimen)-use of SOF + daclatasvir (DCV) for non-cirrhotic patients and ledipasvir (LDV) or DCV with SOF ± ribavirin (RBV) according to the genotype for cirrhotic patients; II-use of SOF + DCV for non-cirrhotic patients and use of SOF+VEL for compensated cirrhotic patients (with RBV in decompensated cirrhosis patients) and III-use of SOF+VEL for both non-cirrhotic and compensated cirrhotic patients (with RBV in decompensated cirrhosis patients). The lifetime costs, life-years and QALYs were assessed for each scenario, using a societal perspective. All the future costs and health outcomes were discounted at an annual rate of 3%. Finally, the incremental cost per QALY gained was computed for each of scenario II and III, as compared to scenario I and for scenario III as compared to II. In addition, we evaluated the lifetime costs and QALYs among HCV patients for each of scenario I, II and III against the counterfactual of 'no universal free treatment scheme' scenario which involves patients purchasing care in routine setting of from public and private sector.
Results: Each of the scenarios I, II and III dominate over the no universal free treatment scheme scenario, i.e. have greater QALYs and lesser costs. The use of SOF+VEL only for cirrhotic patients (scenario II) increases QALYs by 0.28 (0.03 to 0.71) per person, and decreases the cost by ₹ 5,946 (₹ 1,198 to ₹ 14,174) per patient, when compared to scenario I. Compared to scenario I, scenario III leads to an increase in QALYs by 0.44 (0.14 to 1.01) per person, and is cost-neutral. While the mean cost difference between scenario III and I is-₹ 2,676 per patient, it ranges from a cost saving of ₹ 14,835 to incurring an extra cost of ₹ 3,456 per patient. For scenario III as compared II, QALYs increase by 0.16 (0.03 to 0.36) per person as well as costs by ₹ 3,086 per patient which ranges from a cost saving of ₹ 1,264 to incurring an extra cost of ₹ 6,344. Shift to scenario II and III increases the program budget by 5.5% and 60% respectively.
Conclusion: Overall, the use of SOF+VEL is highly recommended for the treatment of HCV infection. In comparison to the current practice (scenario I), scenario II is a dominant option. Scenario III is cost-effective as compared to scenario II at a threshold of one-time GDP per capita. If budget is an important constraint, velpatasvir should be given to HCV infected cirrhotic patients. However, if no budget constraint, universal use of velpatasvir for HCV treatment is recommended.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- World Health Organisation. Burden of Viral Hepatitis in the South-East Asia Region. Available at: hepatitis/ ata/hepatitis_data_ statistics/en/ [Accessed on 25 August 2018].
-
- Government of India, World Health Organisation, Institute of Liver and Biliary Sciences. WHO GoI-ILBS National Technical Consultation on Viral Hepatitis—“Towards a National Action Plan for Viral Hepatitis (NAP-VH).” New Delhi: Institute of Liver and Biliary Sciences; 2016. 44 p.
-
- Mukhopadhya A. Hepatitis C in India. J Biosci. 2008;33(4):465–73. - PubMed
-
- Sievert W, Altraif I, Razavi HA, Abdo A, Ahmed EA, Alomair A, et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31(SUPPL. 2):61–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

