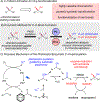
Combined Photoredox/Enzymatic C-H Benzylic Hydroxylations
- PMID: 31465617
- PMCID: PMC6829040
- DOI: 10.1002/anie.201909426
Combined Photoredox/Enzymatic C-H Benzylic Hydroxylations
Retraction in
-
Retraction: Combined Photoredox/Enzymatic C-H Benzylic Hydroxylations.Angew Chem Int Ed Engl. 2023 Sep 25;62(39):e202311469. doi: 10.1002/anie.202311469. Epub 2023 Aug 24. Angew Chem Int Ed Engl. 2023. PMID: 37616507 Free PMC article. No abstract available.
Abstract
Chemical transformations that install heteroatoms into C-H bonds are of significant interest because they streamline the construction of value-added small molecules. Direct C-H oxyfunctionalization, or the one step conversion of a C-H bond to a C-O bond, could be a highly enabling transformation due to the prevalence of the resulting enantioenriched alcohols in pharmaceuticals and natural products,. Here we report a single-flask photoredox/enzymatic process for direct C-H hydroxylation that proceeds with broad reactivity, chemoselectivity and enantioselectivity. This unified strategy advances general photoredox and enzymatic catalysis synergy and enables chemoenzymatic processes for powerful and selective oxidative transformations.
Keywords: C−H oxidation; chemoenzymatic catalysis; chiral alcohols; ketoreductase; photoredox catalysis.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
References
-
- Kille S, Zilly FE, Acevedo JP, Reetz MT, Nat. Chem. 2011, 3, 738; - PubMed
- Kudrik EV, Afanasiev P, Alvarez LX, Dubourdeaux P, Clémancey M, Latour J-M, Blondin G, Bouchu D, Albrieux F, Nefedov SE, Sorokin AB, Nat. Chem. 2012, 4, 1024; - PubMed
- Wencel-Delord J, Glorius F, Nat. Chem. 2013, 5, 369; - PubMed
- Zhang W, Wang F, McCann SD, Wang D, Chen P, Stahl SS, Liu G, Science 2016, 353, 1014; - PMC - PubMed
- Zhang W, Fernández-Fueyo E, Ni Y, van Schie M, Gacs J, Renirie R, Wever R, Mutti FG, Rother D, Alcalde M, Hollmann F, Nat. Catal. 2018, 1, 55–62. - PMC - PubMed
-
- Devine PN, Howard RM, Kumar R, Thompson MP, Truppo MD, Turner NJ, Nat. Rev. Chem. 2018, 2, 409–421;
- van Sch MMCH, Zhang W, Tieves F, Choi DS, Park CB, Burek BO, Bloh JZ, Arends IWCE, Paul CE, Alcalde M, Hollmann F, ACS. Catal. 2019, 10.1021/acscatal.9b01341,7409-7417. - DOI
-
- Choudary BM, Prasad AD, Bhuma V, Swapna V, J. Org. Chem. 1992, 57, 5841–5844;
- Shaabani A, Lee DG, Tetrahedron Lett. 2001, 42, 5833–5836;
- Shaabani A, Mirzaei P, Naderi S, Lee DG, Tetrahedron 2004, 60, 11415–11420.
-
- Baran PS, Zhong Y-L, J. Am. Chem. Soc. 2001, 123, 3183–3185; - PubMed
- Lee JM, Park EJ, Cho SH, Chang S, J. Am. Chem. Soc. 2008, 130, 7824–7825; - PubMed
- Chen MS, White MC, Science 2010, 327, 566; - PubMed
- Xia J-B, Cormier KW, Chen C, Chem. Sci. 2012, 3, 2240–2245; - PMC - PubMed
- Howell JM, Feng K, Clark JR, Trzepkowski LJ, White MC, J. Am. Chem. Soc. 2015, 137, 14590–14593; - PMC - PubMed
- Gensch T, Hopkinson MN, Glorius F, Wencel-Delord J, Chem. Soc. Rev. 2016, 45, 2900–2936; - PubMed
- Gini A, Brandhofer T, Mancheno OG, Org. Biomol. Chem. 2017, 15, 1294–1312; - PubMed
- Nanjo T, de Lucca EC, White MC, J. Am. Chem. Soc. 2017, 139, 14586–14591; - PMC - PubMed
- Li G-X, Morales-Rivera CA, Gao F, Wang Y, He G, Liu P, Chen G, Chem. Sci. 2017, 8, 7180–7185; - PMC - PubMed
- White MC, Zhao J, J. Am. Chem. Soc. 2018, 140, 13988–14009. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources