Molecular Therapies for Inherited Retinal Diseases-Current Standing, Opportunities and Challenges
- PMID: 31466352
- PMCID: PMC6770110
- DOI: 10.3390/genes10090654
Molecular Therapies for Inherited Retinal Diseases-Current Standing, Opportunities and Challenges
Abstract
Inherited retinal diseases (IRDs) are both genetically and clinically highly heterogeneous and have long been considered incurable. Following the successful development of a gene augmentation therapy for biallelic RPE65-associated IRD, this view has changed. As a result, many different therapeutic approaches are currently being developed, in particular a large variety of molecular therapies. These are depending on the severity of the retinal degeneration, knowledge of the pathophysiological mechanism underlying each subtype of IRD, and the therapeutic target molecule. DNA therapies include approaches such as gene augmentation therapy, genome editing and optogenetics. For some genetic subtypes of IRD, RNA therapies and compound therapies have also shown considerable therapeutic potential. In this review, we summarize the current state-of-the-art of various therapeutic approaches, including the pros and cons of each strategy, and outline the future challenges that lie ahead in the combat against IRDs.
Keywords: DNA therapies; IRD; RNA therapies; clinical trials; compound therapies; inherited retinal diseases.
Conflict of interest statement
I.V.D. declares no conflict of interest. A.G. and R.W.J.C. are inventors of several patents related to work cited in this review based on splicing modulation for
Figures
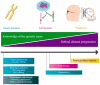
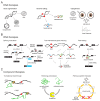
References
-
- Khan M., Fadaie Z., Cornelis S.S., Cremers F.P., Roosing S. Identification and Analysis of Genes Associated with Inherited Retinal Diseases. Methods Mol. Biol. 2019;1834:3–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

