Biocompatibility of Cyclopropylamine-Based Plasma Polymers Deposited at Sub-Atmospheric Pressure on Poly (ε-caprolactone) Nanofiber Meshes
- PMID: 31466357
- PMCID: PMC6780329
- DOI: 10.3390/nano9091215
Biocompatibility of Cyclopropylamine-Based Plasma Polymers Deposited at Sub-Atmospheric Pressure on Poly (ε-caprolactone) Nanofiber Meshes
Abstract
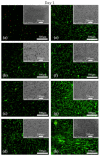
In this work, cyclopropylamine (CPA) monomer was plasma-polymerized on poly (ε-caprolactone) nanofiber meshes using various deposition durations to obtain amine-rich surfaces in an effort to improve the cellular response of the meshes. Scanning electron microscopy and X-ray photoelectron spectroscopy (XPS) were used to investigate the surface morphology and surface chemical composition of the PCL samples, respectively. The measured coating thickness was found to linearly increase with deposition duration at a deposition rate of 0.465 nm/s. XPS analysis revealed that plasma exposure time had a considerable effect on the surface N/C and O/C ratio as well as on amino grafting efficiency and amino selectivity. In addition, cell studies showed that cell adhesion and proliferation significantly improved for all coated samples.
Keywords: biomaterials; cell adhesion; nanofibers; non-thermal plasma; plasma polymerisation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Patterson J., Martino M.M., Hubbell J.A. Biomimetic materials in tissue engineering. Mater. Today. 2010;13:14–22. doi: 10.1016/S1369-7021(10)70013-4. - DOI
-
- Asadian M., Onyshchenko I., Thukkaram M., Saadat P., Tabaei E., Van Guyse J., Cools P., Declercq H., Hoogenboom R., Morent R., et al. Effects of a dielectric barrier discharge (DBD) treatment on chitosan/polyethylene oxide nano fibers and their cellular interactions. Carbohydr. Polym. 2018;201:402–415. doi: 10.1016/j.carbpol.2018.08.092. - DOI - PubMed
-
- Asadian M., Onyshchenko I., Thiry D., Cools P., Declercq H., Snyders R., Morent R., Geyter N. De Applied Surface Science Thiolation of polycaprolactone (PCL) nanofibers by inductively coupled plasma (ICP) polymerization: Physical, chemical and biological properties. Appl. Surf. Sci. 2019;479:942–952. doi: 10.1016/j.apsusc.2019.02.178. - DOI
-
- Asadian M., Dhaenens M., Onyshchenko I., De Waele S., Declercq H., Cools P., Devreese B., Deforce D., Morent R., De Geyter N. Plasma Functionalization of Polycaprolactone Nanofibers Changes Protein Interactions with Cells, Resulting in Increased Cell Viability. ACS Appl. Mater. Interfaces. 2018;10:41962–41977. doi: 10.1021/acsami.8b14995. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

