Synthetic Blocks for Bone Regeneration: A Systematic Review and Meta-Analysis
- PMID: 31466409
- PMCID: PMC6747264
- DOI: 10.3390/ijms20174221
Synthetic Blocks for Bone Regeneration: A Systematic Review and Meta-Analysis
Abstract
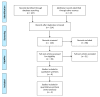
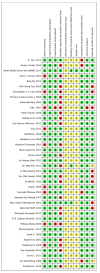
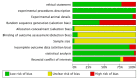
This systematic review is aimed at evaluating the effectiveness of synthetic block materials for bone augmentation in preclinical in vivo studies. An electronic search was performed on Pubmed, Scopus, EMBASE. Articles selected underwent risk-of-bias assessment. The outcomes were: new bone formation and residual graft with histomorphometry, radiographic bone density, soft tissue parameters, complications. Meta-analysis was performed to compare new bone formation in test (synthetic blocks) vs. control group (autogenous blocks or spontaneous healing). The search yielded 214 articles. After screening, 39 studies were included, all performed on animal models: rabbits (n = 18 studies), dogs (n = 4), rats (n = 7), minipigs (n = 4), goats (n = 4), and sheep (n = 2). The meta-analysis on rabbit studies showed significantly higher new bone formation for synthetic blocks with respect to autogenous blocks both at four-week (mean difference (MD): 5.91%, 95% confidence intervals (CI): 1.04, 10.79%, p = 0.02) and at eight-week healing (MD: 4.44%, 95% CI: 0.71, 8.17%, p = 0.02). Other animal models evidenced a trend for better outcomes with synthetic blocks, though only based on qualitative analysis. Synthetic blocks may represent a viable resource in bone regenerative surgery for achieving new bone formation. Differences in the animal models, the design of included studies, and the bone defects treated should be considered when generalizing the results. Clinical studies are needed to confirm the effectiveness of synthetic blocks in bone augmentation procedures.
Keywords: animal models; biomaterials; block graft; bone graft; bone regeneration; bone substitutes; histological analysis; synthetic biomaterials; systematic review.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Chiapasco M., Casentini P., Zaniboni M. Bone augmentation procedures in implant dentistry. Int. J. Oral Maxillofac. Implant. 2009;24:237–259. - PubMed
-
- Jensen S.S., Terheyden H. Bone augmentation procedures in localized defects in the alveolar ridge: Clinical results with different bone grafts and bone-substitute materials. Int. J. Oral Maxillofac. Implant. 2009;24:218–236. - PubMed
-
- Urban I.A., Jovanovic S.A., Lozada J.L. Vertical ridge augmentation using guided bone regeneration (GBR) in three clinical scenarios prior to implant placement: A retrospective study of 35 patients 12 to 72 months after loading. Int. J. Oral Maxillofac. Implant. 2009;24:502–510. - PubMed
-
- Al-Nawas B., Schiegnitz E. Augmentation procedures using bone substitute materials or autogenous bone - a systematic review and meta-analysis. Eur. J. Oral Implant. 2014;7(Suppl. 2):S219–S234. - PubMed

