CDK5 Inhibitor Downregulates Mcl-1 and Sensitizes Pancreatic Cancer Cell Lines to Navitoclax
- PMID: 31467029
- PMCID: PMC6726458
- DOI: 10.1124/mol.119.116855
CDK5 Inhibitor Downregulates Mcl-1 and Sensitizes Pancreatic Cancer Cell Lines to Navitoclax
Abstract
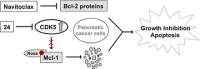
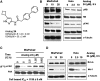
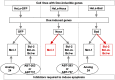
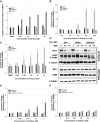
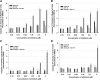
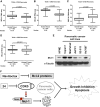
Developing small molecules that indirectly regulate Mcl-1 function has attracted a lot of attention in recent years. Here, we report the discovery of an aminopyrazole, 2-([1,1'-biphenyl]-4-yl)-N-(5-cyclobutyl-1H-pyrazol-3-yl)acetamide (analog 24), which selectively inhibited cyclin-dependent kinase (CDK) 5 over CDK2 in cancer cell lines. We also show that analog 24 reduced Mcl-1 levels in a concentration-dependent manner in cancer cell lines. Using a panel of doxycycline inducible cell lines, we show that CDK5 inhibitor 24 selectively modulates Mcl-1 function while the CDK4/6 inhibitor 6-acetyl-8-cyclopentyl-5-methyl-2-(5-(piperazin-1-yl)pyridin-2-ylamino)pyrido[2,3-day]pyrimidin-7(8H)-one does not. Previous studies using RNA interference and CRISPR showed that concurrent elimination of Bcl-xL and Mcl-1 resulted in induction of apoptosis. In pancreatic cancer cell lines, we show that either CDK5 knockdown or expression of a dominant negative CDK5 results in synergistic induction of apoptosis. Moreover, concurrent pharmacological perturbation of Mcl-1 and Bcl-xL in pancreatic cancer cell lines using a CDK5 inhibitor analog 24 that reduced Mcl-1 levels and 4-(4-{[2-(4-chlorophenyl)-5,5-dimethyl-1-cyclohexen-1-yl]methyl}-1-piperazinyl)-N-[(4-{[(2R)-4-(4-morpholinyl)-1-(phenylsulfanyl)-2-butanyl]amino}-3-[(trifluoromethyl)sulfonyl]phenyl)sulfonyl] benzamide (navitoclax), a Bcl-2/Bcl-xL/Bcl-w inhibitor, resulted in synergistic inhibition of cell growth and induction of apoptosis. In conclusion, we demonstrate targeting CDK5 will sensitize pancreatic cancers to Bcl-2 protein inhibitors. SIGNIFICANCE STATEMENT: Mcl-1 is stabilized by CDK5-mediated phosphorylation in pancreatic ductal adenocarcinoma, resulting in the deregulation of the apoptotic pathway. Thus, genetic or pharmacological targeting of CDK5 sensitizes pancreatic cancers to Bcl-2 inhibitors, such as navitoclax.
Copyright © 2019 by The Author(s).
Figures

Similar articles
-
Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax).Cell Death Dis. 2015 Jan 15;6(1):e1590. doi: 10.1038/cddis.2014.561. Cell Death Dis. 2015. PMID: 25590800 Free PMC article.
-
Bcl-2/Bcl-xL inhibitor navitoclax increases the antitumor effect of Chk1 inhibitor prexasertib by inducing apoptosis in pancreatic cancer cells via inhibition of Bcl-xL but not Bcl-2.Mol Cell Biochem. 2020 Sep;472(1-2):187-198. doi: 10.1007/s11010-020-03796-6. Epub 2020 Jun 21. Mol Cell Biochem. 2020. PMID: 32567031
-
The Bcl-2/xL inhibitor ABT-263 increases the stability of Mcl-1 mRNA and protein in hepatocellular carcinoma cells.Mol Cancer. 2014 Apr 30;13:98. doi: 10.1186/1476-4598-13-98. Mol Cancer. 2014. PMID: 24779770 Free PMC article.
-
JAK2V617F drives Mcl-1 expression and sensitizes hematologic cell lines to dual inhibition of JAK2 and Bcl-xL.PLoS One. 2015 Mar 17;10(3):e0114363. doi: 10.1371/journal.pone.0114363. eCollection 2015. PLoS One. 2015. PMID: 25781882 Free PMC article.
-
Bcl-xL inhibition by molecular-targeting drugs sensitizes human pancreatic cancer cells to TRAIL.Oncotarget. 2015 Dec 8;6(39):41902-15. doi: 10.18632/oncotarget.5881. Oncotarget. 2015. PMID: 26506422 Free PMC article.
Cited by
-
Combination Therapy of Navitoclax with Chemotherapeutic Agents in Solid Tumors and Blood Cancer: A Review of Current Evidence.Pharmaceutics. 2021 Aug 28;13(9):1353. doi: 10.3390/pharmaceutics13091353. Pharmaceutics. 2021. PMID: 34575429 Free PMC article. Review.
-
Necroptosis, pyroptosis and apoptosis: an intricate game of cell death.Cell Mol Immunol. 2021 May;18(5):1106-1121. doi: 10.1038/s41423-020-00630-3. Epub 2021 Mar 30. Cell Mol Immunol. 2021. PMID: 33785842 Free PMC article. Review.
-
Inhibitors, PROTACs and Molecular Glues as Diverse Therapeutic Modalities to Target Cyclin-Dependent Kinase.Cancers (Basel). 2021 Nov 2;13(21):5506. doi: 10.3390/cancers13215506. Cancers (Basel). 2021. PMID: 34771669 Free PMC article. Review.
-
Synergistic activity of ABT-263 and ONC201/TIC10 against solid tumor cell lines is associated with suppression of anti-apoptotic Mcl-1, BAG3, pAkt, and upregulation of pro-apoptotic Noxa and Bax cleavage during apoptosis.Am J Cancer Res. 2023 Jan 15;13(1):307-325. eCollection 2023. Am J Cancer Res. 2023. PMID: 36777502 Free PMC article.
-
Knockdown of Myeloid Cell Leukemia-1 by MicroRNA-101 Increases Sensitivity of A549 Lung Cancer Cells to Etoposide.Iran J Med Sci. 2021 Jul;46(4):298-307. doi: 10.30476/ijms.2020.83173.1203. Iran J Med Sci. 2021. PMID: 34305242 Free PMC article.
References
-
- Byth KF, Thomas A, Hughes G, Forder C, McGregor A, Geh C, Oakes S, Green C, Walker M, Newcombe N, et al. (2009) AZD5438, a potent oral inhibitor of cyclin-dependent kinases 1, 2, and 9, leads to pharmacodynamic changes and potent antitumor effects in human tumor xenografts. Mol Cancer Ther 8:1856–1866. - PubMed
-
- Campani D, Esposito I, Boggi U, Cecchetti D, Menicagli M, De Negri F, Colizzi L, Del Chiaro M, Mosca F, Fornaciari G, et al. (2001) Bcl-2 expression in pancreas development and pancreatic cancer progression. J Pathol 194:444–450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

