Multifaceted HIV integrase functionalities and therapeutic strategies for their inhibition
- PMID: 31467082
- PMCID: PMC6791320
- DOI: 10.1074/jbc.REV119.006901
Multifaceted HIV integrase functionalities and therapeutic strategies for their inhibition
Abstract
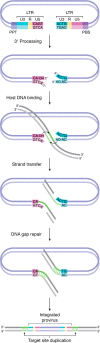
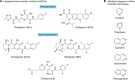
Antiretroviral inhibitors that are used to manage HIV infection/AIDS predominantly target three enzymes required for virus replication: reverse transcriptase, protease, and integrase. Although integrase inhibitors were the last among this group to be approved for treating people living with HIV, they have since risen to the forefront of treatment options. Integrase strand transfer inhibitors (INSTIs) are now recommended components of frontline and drug-switch antiretroviral therapy formulations. Integrase catalyzes two successive magnesium-dependent polynucleotidyl transferase reactions, 3' processing and strand transfer, and INSTIs tightly bind the divalent metal ions and viral DNA end after 3' processing, displacing from the integrase active site the DNA 3'-hydroxyl group that is required for strand transfer activity. Although second-generation INSTIs present higher barriers to the development of viral drug resistance than first-generation compounds, the mechanisms underlying these superior barrier profiles are incompletely understood. A separate class of HIV-1 integrase inhibitors, the allosteric integrase inhibitors (ALLINIs), engage integrase distal from the enzyme active site, namely at the binding site for the cellular cofactor lens epithelium-derived growth factor (LEDGF)/p75 that helps to guide integration into host genes. ALLINIs inhibit HIV-1 replication by inducing integrase hypermultimerization, which precludes integrase binding to genomic RNA and perturbs the morphogenesis of new viral particles. Although not yet approved for human use, ALLINIs provide important probes that can be used to investigate the link between HIV-1 integrase and viral particle morphogenesis. Herein, I review the mechanisms of retroviral integration as well as the promises and challenges of using integrase inhibitors for HIV/AIDS management.
Keywords: AIDS; HIV/AIDS; antiretroviral therapy; intasome; integrase; integrase strand transfer inhibitor; integration; microbiology; polynucleotidyl transferase; retrovirus; viral DNA; virology; virus structure.
© 2019 Engelman.
Conflict of interest statement
The author has received fees from ViiV Healthcare Co. within the past 12 months
Figures


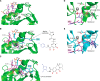

References
-
- Engelman A. (2010) Reverse transcription and integration. In Retroviruses: Molecular Biology, Genomics and Pathogenesis (Kurth R., and Bannert N., eds) pp. 129–159, Caister Academic Press, Norfolk, UK
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

