Notch3 promotes prostate cancer-induced bone lesion development via MMP-3
- PMID: 31467432
- PMCID: PMC6938550
- DOI: 10.1038/s41388-019-0977-1
Notch3 promotes prostate cancer-induced bone lesion development via MMP-3
Abstract
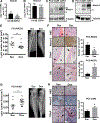
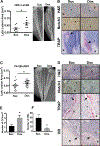
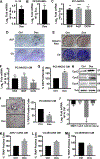
Prostate cancer metastases primarily localize in the bone where they induce a unique osteoblastic response. Elevated Notch activity is associated with high-grade disease and metastasis. To address how Notch affects prostate cancer bone lesions, we manipulated Notch expression in mouse tibia xenografts and monitored tumor growth, lesion phenotype, and the bone microenvironment. Prostate cancer cell lines that induce mixed osteoblastic lesions in bone expressed 5-6 times more Notch3, than tumor cells that produce osteolytic lesions. Expression of active Notch3 (NICD3) in osteolytic tumors reduced osteolytic lesion area and enhanced osteoblastogenesis, while loss of Notch3 in osteoblastic tumors enhanced osteolytic lesion area and decreased osteoblastogensis. This was accompanied by a respective decrease and increase in the number of active osteoclasts and osteoblasts at the tumor-bone interface, without any effect on tumor proliferation. Conditioned medium from NICD3-expressing cells enhanced osteoblast differentiation and proliferation in vitro, while simultaneously inhibiting osteoclastogenesis. MMP-3 was specifically elevated and secreted by NICD3-expressing tumors, and inhibition of MMP-3 rescued the NICD3-induced osteoblastic phenotypes. Clinical osteoblastic bone metastasis samples had higher levels of Notch3 and MMP-3 compared with patient matched visceral metastases or osteolytic metastasis samples. We identified a Notch3-MMP-3 axis in human prostate cancer bone metastases that contributes to osteoblastic lesion formation by blocking osteoclast differentiation, while also contributing to osteoblastogenesis. These studies define a new role for Notch3 in manipulating the tumor microenvironment in bone metastases.
Conflict of interest statement
CONFLICT OF INTEREST:
The authors declare no competing financial or conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

