Baicalin Protects against Thrombin-Induced Cell Injury in Human Umbilical Vein Endothelial Cells
- PMID: 31467874
- PMCID: PMC6699368
- DOI: 10.1155/2019/2187306
Baicalin Protects against Thrombin-Induced Cell Injury in Human Umbilical Vein Endothelial Cells
Abstract
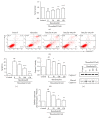
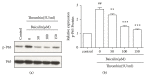
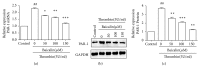
Thrombin plays a pivotal role in the pathogenesis of atherosclerosis. Baicalin, an active flavonoid compound, was shown to attenuate the development of atherosclerosis, but the mechanism remains elusive. In the present study, the role and mechanism of baicalin in thrombin-induced cell injury was investigated in human umbilical vein endothelial cells (HUVECs). Our results showed that baicalin significantly reduced thrombin-induced apoptosis of HUVECs. Additional experiments showed that baicalin inhibited thrombin-induced NF-κB activation and PAR-1 expression. In addition, baicalin decreased thrombin-induced PAR-1 expression by inhibiting ERK pathway. These results indicated that baicalin has protective effects on thrombin-induced cell injury in HUVECs possibly through inhibition of PAR-1 expression and its downstream NF-κB activation, which was mediated by ERK1/2 activation.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Baicalin protects against thrombin induced cell injury in SH-SY5Y cells.Int J Clin Exp Pathol. 2015 Nov 1;8(11):14021-7. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26823714 Free PMC article.
-
Dihydromyricetin protects human umbilical vein endothelial cells from injury through ERK and Akt mediated Nrf2/HO-1 signaling pathway.Apoptosis. 2017 Aug;22(8):1013-1024. doi: 10.1007/s10495-017-1381-3. Apoptosis. 2017. PMID: 28612103
-
Baicalin alleviates atherosclerosis by relieving oxidative stress and inflammatory responses via inactivating the NF-κB and p38 MAPK signaling pathways.Biomed Pharmacother. 2018 Jan;97:1673-1679. doi: 10.1016/j.biopha.2017.12.024. Epub 2017 Dec 8. Biomed Pharmacother. 2018. PMID: 29793330
-
Thrombin/PAR-1 activation induces endothelial damages via NLRP1 inflammasome in gestational diabetes.Biochem Pharmacol. 2020 May;175:113849. doi: 10.1016/j.bcp.2020.113849. Epub 2020 Feb 12. Biochem Pharmacol. 2020. PMID: 32059841
-
Exploring the Cardiovascular Protective Effects of Baicalin: A Pathway to New Therapeutic Insights.Curr Top Med Chem. 2025;25(2):163-171. doi: 10.2174/0115680266347503241008075106. Curr Top Med Chem. 2025. PMID: 39390834 Review.
Cited by
-
Morin, as a natural flavonoid, provides promising influences against cardiovascular diseases.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun;398(6):6293-6310. doi: 10.1007/s00210-024-03783-4. Epub 2025 Jan 14. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39808314
-
The Pharmacological Effects of Silver Nanoparticles Functionalized with Eptifibatide on Platelets and Endothelial Cells.Int J Nanomedicine. 2022 Sep 19;17:4383-4400. doi: 10.2147/IJN.S373691. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36164554 Free PMC article.
-
Research Progress in In Vitro Screening Techniques for Natural Antithrombotic Medicines.Pharmaceuticals (Basel). 2025 Jan 21;18(2):137. doi: 10.3390/ph18020137. Pharmaceuticals (Basel). 2025. PMID: 40005952 Free PMC article. Review.
-
Pharmacological mechanisms by which baicalin ameliorates cardiovascular disease.Front Pharmacol. 2024 Aug 9;15:1415971. doi: 10.3389/fphar.2024.1415971. eCollection 2024. Front Pharmacol. 2024. PMID: 39185317 Free PMC article. Review.
-
Baicalin and Baicalein Enhance Cytotoxicity, Proapoptotic Activity, and Genotoxicity of Doxorubicin and Docetaxel in MCF-7 Breast Cancer Cells.Molecules. 2024 May 25;29(11):2503. doi: 10.3390/molecules29112503. Molecules. 2024. PMID: 38893380 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

