The Lysine Demethylase KDM5B Regulates Islet Function and Glucose Homeostasis
- PMID: 31467927
- PMCID: PMC6701283
- DOI: 10.1155/2019/5451038
The Lysine Demethylase KDM5B Regulates Islet Function and Glucose Homeostasis
Abstract
Aims: Posttranslational modifications of histones and transcription factors regulate gene expression and are implicated in beta-cell failure and diabetes. We have recently shown that preserving H3K27 and H3K4 methylation using the lysine demethylase inhibitor GSK-J4 reduces cytokine-induced destruction of beta-cells and improves beta-cell function. Here, we investigate the therapeutic potential of GSK-J4 to prevent diabetes development and examine the importance of H3K4 methylation for islet function.
Materials and methods: We used two mouse models of diabetes to investigate the therapeutic potential of GSK-J4. To clarify the importance of H3K4 methylation, we characterized a mouse strain with knockout (KO) of the H3K4 demethylase KDM5B.
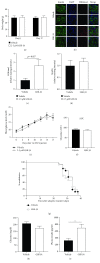
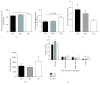
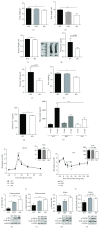
Results: GSK-J4 administration failed to prevent the development of experimental diabetes induced by multiple low-dose streptozotocin or adoptive transfer of splenocytes from acutely diabetic NOD to NODscid mice. KDM5B-KO mice were growth retarded with altered body composition, had low IGF-1 levels, and exhibited reduced insulin secretion. Interestingly, despite secreting less insulin, KDM5B-KO mice were able to maintain normoglycemia following oral glucose tolerance test, likely via improved insulin sensitivity, as suggested by insulin tolerance testing and phosphorylation of proteins belonging to the insulin signaling pathway. When challenged with high-fat diet, KDM5B-deficient mice displayed similar weight gain and insulin sensitivity as wild-type mice.
Conclusion: Our results show a novel role of KDM5B in metabolism, as KDM5B-KO mice display growth retardation and improved insulin sensitivity.
Conflict of interest statement
We declare no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

