Functionalized magnetic particles for water treatment
- PMID: 31467994
- PMCID: PMC6710533
- DOI: 10.1016/j.heliyon.2019.e02325
Functionalized magnetic particles for water treatment
Abstract
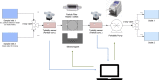
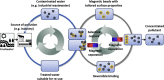
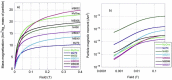
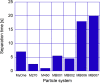
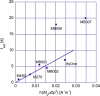
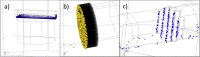
In this study, we have taken the concept of water treatment by functionalized magnetic particles one step forward by integrating the technology into a complete proof of concept, which included the preparation of surface modified beads, their use as highly selective absorbents for heavy metals ions (Zinc, Nickel), and their performance in terms of magnetic separation. The separation characteristics were studied both through experiments and by simulations. The data gathered from these experimental works enabled the elaboration of various scenarios for Life Cycle Analysis (LCA). The LCA showed that the environmental impact of the system is highly dependent on the recovery rate of the magnetic particles. The absolute impact on climate change varied significantly among the scenarios studied and the recovery rates. The results support the hypothesis that chelation specificity, magnetic separation and bead recovery should be optimized to specific targets and applications.
Keywords: Chemical engineering; Environmental science; Life cycle assessment; Magnetic particle; Materials chemistry; Nanotechnology; Pollutant; Water treatment.
Figures


References
-
- Bordes R., Holmberg K. Amino acid-based surfactants – do they deserve more attention? Adv. Colloid Interface Sci. 2015;222:79–91. - PubMed
-
- Bordes R., Tropsch J., Holmberg K. Counterion specificity of surfactants based on dicarboxylic amino acids. J. Colloid Interface Sci. 2009;338:529–536. - PubMed
-
- Cleuvers M. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol. Lett. 2003;142:185–194. - PubMed
-
- Dixit R., Malaviya D., Pandiyan K., Singh U.B., Sahu A., Shukla R., Singh B.P., Rai J.P., Sharma P.K., Lade H. Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability. 2015;7:2189–2212.
LinkOut - more resources
Full Text Sources
Research Materials

