Whole cell biosynthesis of 1-methyl-3-phenylpropylamine and 2-amino-1,3,4-butanetriol using Komagataella phaffii (Pichia pastoris) strain BG-10 engineered with a transgene encoding Chromobacterium violaceum ω-transaminase
- PMID: 31467995
- PMCID: PMC6710532
- DOI: 10.1016/j.heliyon.2019.e02338
Whole cell biosynthesis of 1-methyl-3-phenylpropylamine and 2-amino-1,3,4-butanetriol using Komagataella phaffii (Pichia pastoris) strain BG-10 engineered with a transgene encoding Chromobacterium violaceum ω-transaminase
Abstract
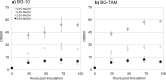
We have engineered strain BG-10 of the methylotrophic yeast Komagataella phaffii for use as an effective whole cell biocatalyst. We introduced into the yeast a transgene encoding a Chromobacterium violaceum ω-transaminase for transcription in response to methanol induction. The strain was then assessed with respect to its growth performance and biotransformation of a fed ketoalcohol substrate to an amino-alcohol. In the resultant strain, BG-TAM, methanol induction did not compromise cell growth. Successful bioconversion of fed substrates to the by-product, acetophenone, indicated transaminase activity in shake flask-cultivated BG-TAM cells. We then used bioreactor cultivation to exploit the high levels of biomass achievable by Komagataella phaffii. In a 900 μL reaction the BG-TAM strain at OD600 = 1024 achieved up to 0.41 mol mol-1 (molproduct molsubstrate -1) yield on substrate (Yp/s) for production of 1-methyl-3-phenylpropylamine and a space time yield (STY) of 0.29 g L-1 h-1 for production of 2-amino-1,3,4-butanetriol. We have shown that transamination, an important step for bespoke synthesis of small molecule medicines, is biologically realisable using enzymes with a broad substrate range, such as ω-transaminases, within living yeast cells that are fed low-cost substrates for bioconversion.
Keywords: Bioengineering; Biotechnology; Chemical engineering; Komagataella phaffii; Pichia pastoris; Transaminase; Whole cell biocatalyst.
Figures



References
-
- Ager D.J., Prakash I., Schaad D.R. 1,2-Amino alcohols and their heterocyclic derivatives as chiral auxiliaries in asymmetric synthesis. Chem. Rev. 1996;96:835–876. - PubMed
-
- Bea H.S., Seo Y.M., Cha M.H., Kim B.G., Yun H. Kinetic resolution of α-methylbenzylamine by recombinant Pichia pastoris expressing ω-transaminase. Biotechnol. Bioproc. Eng. 2010;15:429–434.
-
- Braun-Galleani S., Henriquez M.J., Nesbeth D.N. 2018. Codon Optimisation of a Chromobacterium Violaceum ω-transaminase for Expression in Komagataella Phaffii (Pichia pastoris)
LinkOut - more resources
Full Text Sources

