Notch signalling is a potential resistance mechanism of progenitor cells within patient-derived prostate cultures following ROS-inducing treatments
- PMID: 31468514
- PMCID: PMC7003772
- DOI: 10.1002/1873-3468.13589
Notch signalling is a potential resistance mechanism of progenitor cells within patient-derived prostate cultures following ROS-inducing treatments
Abstract
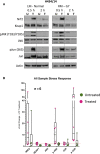
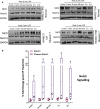
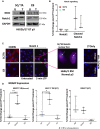
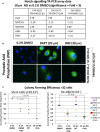
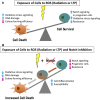
Low Temperature Plasma (LTP) generates reactive oxygen and nitrogen species, causing cell death, similarly to radiation. Radiation resistance results in tumour recurrence, however mechanisms of LTP resistance are unknown. LTP was applied to patient-derived prostate epithelial cells and gene expression assessed. A typical global oxidative response (AP-1 and Nrf2 signalling) was induced, whereas Notch signalling was activated exclusively in progenitor cells. Notch inhibition induced expression of prostatic acid phosphatase (PAP), a marker of prostate epithelial cell differentiation, whilst reducing colony forming ability and preventing tumour formation. Therefore, if LTP is to be progressed as a novel treatment for prostate cancer, combination treatments should be considered in the context of cellular heterogeneity and existence of cell type-specific resistance mechanisms.
Keywords: Low temperature plasma; Notch signalling; Therapy resistance; progenitor cells; prostate cancer; reactive oxygen species.
© 2019 The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures




References
-
- Kang SU, Cho JH, Chang JW, Shin YS, Kim KI, Park JK, Yang SS, Lee JS, Moon E, Lee K et al (2014) Nonthermal plasma induces head and neck cancer cell death: the potential involvement of mitogen‐activated protein kinase‐dependent mitochondrial reactive oxygen species. Cell Death Dis 5, e1056. - PMC - PubMed
-
- Klaunig JE, Kamendulis LM and Hocevar BA (2010) Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol 38, 96–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

