Early prediction of phenotypic severity in Citrullinemia Type 1
- PMID: 31469252
- PMCID: PMC6764635
- DOI: 10.1002/acn3.50886
Early prediction of phenotypic severity in Citrullinemia Type 1
Abstract
Objective: Citrullinemia type 1 (CTLN1) is an inherited metabolic disease affecting the brain which is detectable by newborn screening. The clinical spectrum is highly variable including individuals with lethal hyperammonemic encephalopathy in the newborn period and individuals with a mild-to-moderate or asymptomatic disease course. Since the phenotypic severity has not been predictable early during the disease course so far, we aimed to design a reliable disease prediction model.
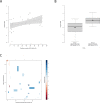
Methods: We used a newly established mammalian biallelic expression system to determine residual enzymatic activity of argininosuccinate synthetase 1 (ASS1; OMIM #215700) in 71 individuals with CTLN1, representing 48 ASS1 gene variants and 50 different, mostly compound heterozygous combinations in total. Residual enzymatic ASS1 activity was correlated to standardized biochemical and clinical endpoints available from the UCDC and E-IMD databases.
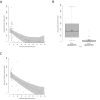
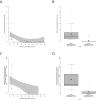
Results: Residual enzymatic ASS1 activity correlates with peak plasma ammonium and L-citrulline concentrations at initial presentation. Individuals with 8% of residual enzymatic ASS1 activity or less had more frequent and more severe hyperammonemic events and lower cognitive function than those above 8%, highlighting that residual enzymatic ASS1 activity allows reliable severity prediction. Noteworthy, empiric clinical practice of affected individuals is in line with the predicted disease severity supporting the notion of a risk stratification-based guidance of therapeutic decision-making based on residual enzymatic ASS1 activity in the future.
Interpretation: Residual enzymatic ASS1 activity reliably predicts the phenotypic severity in CTLN1. We propose a new severity-adjusted classification system for individuals with CTLN1 based on the activity results of the newly established biallelic expression system.
© 2019 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association.
Conflict of interest statement
SK receives funding from Horizon Pharma Ireland Limited for the European Post‐Authorization Registry for Ravicti® (glycerol phenylbutyrate) oral liquid in partnership with the E‐IMD (RRPE) (EU PAS Register no. EUPAS17267;
Figures


References
-
- Kolker S, Garcia‐Cazorla A, Valayannopoulos V, et al. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 1: the initial presentation. J Inherit Metab Dis 2015;38:1041–1057. - PubMed
-
- Kolker S, Valayannopoulos V, Burlina AB, et al. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 2: the evolving clinical phenotype. J Inherit Metab Dis 2015;38:1059–1074. - PubMed
-
- Burgard P, Kolker S, Haege G, et al. Neonatal mortality and outcome at the end of the first year of life in early onset urea cycle disorders–review and meta‐analysis of observational studies published over more than 35 years. J Inherit Metab Dis 2016;39:219–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

