Effect of Sequential or Active Choice for Colorectal Cancer Screening Outreach: A Randomized Clinical Trial
- PMID: 31469393
- PMCID: PMC6724166
- DOI: 10.1001/jamanetworkopen.2019.10305
Effect of Sequential or Active Choice for Colorectal Cancer Screening Outreach: A Randomized Clinical Trial
Abstract
Importance: Colonoscopy and fecal immunochemical testing (FIT) are considered top-tier tests for colorectal cancer (CRC) screening. Behavioral economic insights about "choice architecture" suggest that participation could be influenced by how people are presented test options.
Objective: To investigate response rates for offering colonoscopy only compared with sequential choice (colonoscopy and then FIT) or active choice (colonoscopy or FIT) through mailed outreach.
Design, setting, and participants: Three-arm pragmatic randomized clinical trial conducted between November 14, 2017, and May 14, 2018. The setting was primary care practices at an academic health system. Patients aged 50 to 74 years with at least 2 primary care visits in the 2-year preenrollment period were included if they were eligible but not up to date on CRC screening.
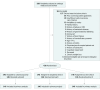
Interventions: Eligible patients received mailed outreach about CRC screening. Equal numbers of eligible patients were randomly assigned to 3 outreach groups to receive mailings about CRC screening with the following options: (1) direct phone number to call for scheduling colonoscopy (colonoscopy only), (2) direct phone number to call for colonoscopy and a mailed FIT kit if no response within 4 weeks (sequential choice), or (3) direct phone number to call for colonoscopy and a mailed FIT kit offered at the same time (active choice).
Main outcomes and measures: The primary outcome was CRC screening completion (FIT or colonoscopy) within 4 months of initial outreach. The secondary outcomes were CRC screening completion within 6 months of outreach and the choice of colonoscopy as a screening test.
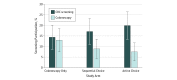
Results: In total, 438 patients were included in the intent-to-treat analysis, with a median age of 56 years (interquartile range, 52-63 years); 55.0% were women. At 4 months, the CRC screening completion rates were 14.4% (95% CI, 8.7%-20.1%) in the colonoscopy-only arm, 17.1% (95% CI, 11.0%-23.2%) in the sequential choice arm, and 19.9% (95% CI, 13.4%-26.4%) in the active choice arm. Neither choice arm achieved a screening rate statistically greater than that in the colonoscopy-alone arm. Among those who completed CRC screening at 4 months, 90.5% (95% CI, 78.0%-103.0%) chose colonoscopy in the colonoscopy-only arm, which was significantly higher than the 52.0% (95% CI, 32.4%-71.6%; P = .005) and 37.9% (95% CI, 20.2%-55.6%; P < .001) in the sequential choice and active choice arms, respectively.
Conclusions and relevance: There was no significant increase in CRC screening when offering sequential or active choice, but there was a lower rate of colonoscopy in the choice arms than in the colonoscopy-only arm. Subtle changes in sequencing or defaults can alter patient decision making related to preventive health.
Trial registration: ClinicalTrials.gov identifier: NCT03246438.
Conflict of interest statement
Figures
Comment in
-
You Should Get Screened for Colon Cancer, Really.JAMA Netw Open. 2019 Aug 2;2(8):e1910452. doi: 10.1001/jamanetworkopen.2019.10452. JAMA Netw Open. 2019. PMID: 31469390 No abstract available.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

