Alkaloid Enantiomers from the Roots of Isatis indigotica
- PMID: 31470525
- PMCID: PMC6749297
- DOI: 10.3390/molecules24173140
Alkaloid Enantiomers from the Roots of Isatis indigotica
Abstract
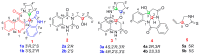
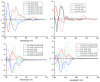
Five pairs of alkaloid enantiomers (1a/1b-5a/5b) were obtained from Isatis indigotica (I. indigotica) roots. Among them, 1a/1b, 2a/2b and 3a/3b were determined as three pairs of new alkaloid enantiomers. Their structures were elucidated by physicochemical properties and spectroscopic methods. The absolute configurations were deduced by comparison of their experimental circular dichroism (CD) and calculated electronic circular dichroism (ECD) spectra, as well as by single-crystal X-ray crystallography using anomalous scattering of Cu Kα radiation. Alkaloids 1a and 1b possess an unpresented carbon skeleton and their putative biosynthetic pathways are discussed. Moreover, all of the alkaloids were tested for their nitric oxide (NO) inhibitory effects in RAW 264.7 cells, and 4a and 4b showed inhibitory effects with IC50 values of 76.97 μM and 65.88 μM, respectively.
Keywords: Isatis indigotica; alkaloid enantiomers; anti-inflammatory activity; structure deduction.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
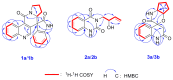


Similar articles
-
Isatis indigotica: from (ethno) botany, biochemistry to synthetic biology.Mol Hortic. 2021 Dec 14;1(1):17. doi: 10.1186/s43897-021-00021-w. Mol Hortic. 2021. PMID: 37789475 Free PMC article. Review.
-
Bisindole alkaloids with nitric oxide inhibitory activities from an alcohol extract of the Isatis indigotica roots.Fitoterapia. 2020 Oct;146:104654. doi: 10.1016/j.fitote.2020.104654. Epub 2020 Jun 2. Fitoterapia. 2020. PMID: 32502502
-
Isatisindigoticanine A, a novel indole alkaloid with an unpresented carbon skeleton from the roots of Isatis tinctoria.Nat Prod Res. 2021 Apr;35(8):1249-1255. doi: 10.1080/14786419.2019.1644632. Epub 2019 Jul 22. Nat Prod Res. 2021. PMID: 31328551
-
Alkaloids from the root of Isatis indigotica.J Nat Prod. 2012 Jun 22;75(6):1167-76. doi: 10.1021/np3002833. Epub 2012 Jun 13. J Nat Prod. 2012. PMID: 22694318
-
A Systemic Review for Ethnopharmacological Studies on Isatis indigotica Fortune: Bioactive Compounds and their Therapeutic Insights.Am J Chin Med. 2022;50(1):161-207. doi: 10.1142/S0192415X22500069. Epub 2022 Feb 9. Am J Chin Med. 2022. PMID: 35139772
Cited by
-
Plants-Derived Biomolecules as Potent Antiviral Phytomedicines: New Insights on Ethnobotanical Evidences against Coronaviruses.Plants (Basel). 2020 Sep 21;9(9):1244. doi: 10.3390/plants9091244. Plants (Basel). 2020. PMID: 32967179 Free PMC article. Review.
-
Alkaloids with Nitric Oxide Inhibitory Activities from the Roots of Isatis tinctoria.Molecules. 2019 Nov 7;24(22):4033. doi: 10.3390/molecules24224033. Molecules. 2019. PMID: 31703370 Free PMC article.
-
Functional Characterization of UDP-Glycosyltransferases Involved in Anti-viral Lignan Glycosides Biosynthesis in Isatis indigotica.Front Plant Sci. 2022 Jun 14;13:921815. doi: 10.3389/fpls.2022.921815. eCollection 2022. Front Plant Sci. 2022. PMID: 35774804 Free PMC article.
-
Isatis indigotica: from (ethno) botany, biochemistry to synthetic biology.Mol Hortic. 2021 Dec 14;1(1):17. doi: 10.1186/s43897-021-00021-w. Mol Hortic. 2021. PMID: 37789475 Free PMC article. Review.
-
Natural Enantiomers: Occurrence, Biogenesis and Biological Properties.Molecules. 2022 Feb 14;27(4):1279. doi: 10.3390/molecules27041279. Molecules. 2022. PMID: 35209066 Free PMC article. Review.
References
-
- Chen M.H., Lin S., Li L., Zhu C.G., Wang X.L., Wang Y.A., Jiang B.Y., Wang S.J., Li Y.H., Jiang J.D. Enantiomers of an indole alkaloid containing unusual dihydrothiopyran and 1,2,4-thiadiazole rings from the root of Isatis indigotica. Org. Lett. 2015;45:1523–7052. doi: 10.1021/ol302660t. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

