Nutritional Stress in Head and Neck Cancer Originating Cell Lines: The Sensitivity of the NRF2-NQO1 Axis
- PMID: 31470592
- PMCID: PMC6769674
- DOI: 10.3390/cells8091001
Nutritional Stress in Head and Neck Cancer Originating Cell Lines: The Sensitivity of the NRF2-NQO1 Axis
Abstract
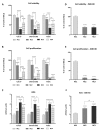
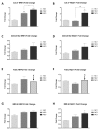
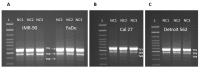
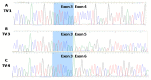
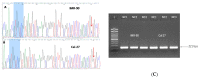
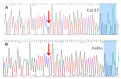
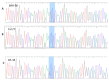
Nutritional stress disturbs the cellular redox-status, which is characterized by the increased generation of reactive oxygen species (ROS). The NRF2-NQO1 axis represents a protective mechanism against ROS. Its strength is cell type-specific. FaDu, Cal 27 and Detroit 562 cells differ with respect to basal NQO1 activity. These cells were grown for 48 hours in nutritional conditions (NC): (a) Low glucose-NC2, (b) no glucose, no glutamine-NC3, (c) no glucose with glutamine-NC4. After determining the viability, proliferation and ROS generation, NC2 and NC3 were chosen for further exploration. These conditions were also applied to IMR-90 fibroblasts. The transcripts/transcript variants of NRF2 and NQO1 were quantified and transcript variants were characterized. The proteins (NRF2, NQO1 and TP53) were analyzed by a western blot in both cellular fractions. Under NC2, the NRF2-NQO1 axis did not appear activated in the cancer cell lines. Under NC3, the NRF2-NQO1axis appeared slightly activated in Detroit 562. There are opposite trends with respect to TP53 nuclear signal when comparing Cal 27 and Detroit 562 to FaDu, under NC2 and NC3. The strong activation of the NRF2-NQO1 axis in IMR-90 resulted in an increased expression of catalytically deficient NQO1, due to NQO1*2/*2 polymorphism (rs1800566). The presented results call for a comprehensive exploration of the stress response in complex biological systems.
Keywords: IMR-90; NQO1 transcript variants; NRF2-NQO1 axis; ROS; TP53 mutation; glucose deprivation; glutamine deprivation; proliferation; rs1800566; viability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

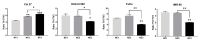

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

