Autograft microskin combined with adipose-derived stem cell enhances wound healing in a full-thickness skin defect mouse model
- PMID: 31470890
- PMCID: PMC6717360
- DOI: 10.1186/s13287-019-1389-4
Autograft microskin combined with adipose-derived stem cell enhances wound healing in a full-thickness skin defect mouse model
Abstract
Objective: Autograft microskin transplantation has been widely used as a skin graft therapy in full-thickness skin defect. However, skin grafting failure can lead to a pathological delay wound healing due to a poor vascularization bed. Considering the active role of adipose-derived stem cell (ADSC) in promoting angiogenesis, we intend to investigate the efficacy of autograft microskin combined with ADSC transplantation for facilitating wound healing in a full-thickness skin defect mouse model.
Material and methods: An in vivo full-thickness skin defect mouse model was used to evaluate the contribution of transplantation microskin and ADSC in wound healing. The angiogenesis was detected by immunohistochemistry staining. In vitro paracrine signaling pathway was evaluated by protein array and Gene Ontology, Kyoto Encyclopedia of Genes and Genomes pathway, and protein-protein interaction network analysis.
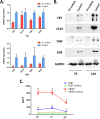
Results: Co-transplantation of microskin and ADSC potentiated the wound healing with better epithelization, smaller scar thickness, and higher angiogenesis (CD31) in the subcutaneous layer. We found both EGF and VEGF cytokines were secreted by microskin in vitro. Additionally, secretome proteomic analysis in a co-culture system of microskin and ADSC revealed that ADSC could secrete a wide range of important molecules to form a reacting network with microskin, including VEGF, IL-6, EGF, uPAR, MCP-3, G-CSF, and Tie-2, which most likely supported the angiogenesis effect as observed.
Conclusion: Overall, we concluded that the use of ADSC partially modulates microskin function and enhances wound healing by promoting angiogenesis in a full-thickness skin defect mouse model.
Keywords: Adipose-derived stem cell; Full-thickness skin defect; Microskin; Secretome; Wound healing.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Wang WZ, Baynosa RC, Zamboni WA. Update on ischemia-reperfusion injury for the plastic surgeon: 2011. Plast Reconstr Surg. 2011;128(6):685e–692e. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

