More than Microtubules: The Structure and Function of the Subpellicular Array in Trypanosomatids
- PMID: 31471215
- PMCID: PMC6783356
- DOI: 10.1016/j.pt.2019.07.008
More than Microtubules: The Structure and Function of the Subpellicular Array in Trypanosomatids
Abstract
The subpellicular microtubule array defines the wide range of cellular morphologies found in parasitic kinetoplastids (trypanosomatids). Morphological studies have characterized array organization, but little progress has been made towards identifying the molecular mechanisms that are responsible for array differentiation during the trypanosomatid life cycle, or the apparent stability and longevity of array microtubules. In this review, we outline what is known about the structure and biogenesis of the array, with emphasis on Trypanosoma brucei, Trypanosoma cruzi, and Leishmania, which cause life-threatening diseases in humans and livestock. We highlight unanswered questions about this remarkable cellular structure that merit new consideration in light of our recently improved understanding of how the 'tubulin code' influences microtubule dynamics to generate complex cellular structures.
Keywords: Trypanosoma; corset; cytokinesis; microtubules; morphology; subpellicular array.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures

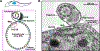


Similar articles
-
The Trypanosoma brucei subpellicular microtubule array is organized into functionally discrete subdomains defined by microtubule associated proteins.PLoS Pathog. 2021 May 19;17(5):e1009588. doi: 10.1371/journal.ppat.1009588. eCollection 2021 May. PLoS Pathog. 2021. PMID: 34010336 Free PMC article.
-
An orphan kinesin in trypanosomes cooperates with a kinetoplastid-specific kinesin to maintain cell morphology by regulating subpellicular microtubules.J Cell Sci. 2012 Sep 1;125(Pt 17):4126-36. doi: 10.1242/jcs.106534. Epub 2012 May 23. J Cell Sci. 2012. PMID: 22623724 Free PMC article.
-
Trypanosomatid Flagellar Pocket from Structure to Function.Trends Parasitol. 2021 Apr;37(4):317-329. doi: 10.1016/j.pt.2020.11.005. Epub 2020 Dec 8. Trends Parasitol. 2021. PMID: 33308952 Review.
-
Cytokinesis in Trypanosoma brucei relies on an orphan kinesin that dynamically crosslinks microtubules.Curr Biol. 2023 Mar 13;33(5):899-911.e5. doi: 10.1016/j.cub.2023.01.035. Epub 2023 Feb 13. Curr Biol. 2023. PMID: 36787745 Free PMC article.
-
The cytoskeleton of trypanosomatid parasites.Annu Rev Microbiol. 1999;53:629-55. doi: 10.1146/annurev.micro.53.1.629. Annu Rev Microbiol. 1999. PMID: 10547703 Review.
Cited by
-
Whole cell reconstructions of Leishmania mexicana through the cell cycle.PLoS Pathog. 2024 Feb 28;20(2):e1012054. doi: 10.1371/journal.ppat.1012054. eCollection 2024 Feb. PLoS Pathog. 2024. PMID: 38416776 Free PMC article.
-
Recombinant antibody against Trypanosoma cruzi from patients with chronic Chagas heart disease recognizes mammalian nervous system.EBioMedicine. 2021 Jan;63:103206. doi: 10.1016/j.ebiom.2020.103206. Epub 2021 Jan 9. EBioMedicine. 2021. PMID: 33429173 Free PMC article.
-
The Trypanosoma brucei subpellicular microtubule array is organized into functionally discrete subdomains defined by microtubule associated proteins.PLoS Pathog. 2021 May 19;17(5):e1009588. doi: 10.1371/journal.ppat.1009588. eCollection 2021 May. PLoS Pathog. 2021. PMID: 34010336 Free PMC article.
-
Novel Cytoskeleton-Associated Proteins in Trypanosoma brucei Are Essential for Cell Morphogenesis and Cytokinesis.Microorganisms. 2021 Oct 27;9(11):2234. doi: 10.3390/microorganisms9112234. Microorganisms. 2021. PMID: 34835360 Free PMC article.
-
Alternate histories of cytokinesis: lessons from the trypanosomatids.Mol Biol Cell. 2020 Nov 15;31(24):2631-2639. doi: 10.1091/mbc.E19-12-0696. Mol Biol Cell. 2020. PMID: 33180676 Free PMC article. Review.
References
-
- Brooker BE (1971) Fine structure of Bodo saltans and Bodo caudatus (Zoomastigophora: Protozoa) and their affinities with the Trypanosomatidae. Bull. Brit. Mus. Nat. Hist 22, 89–102
-
- Angelopoulos E (1970) Pellicular Microtubules in the Family Trypanosomatidae. The Journal of Protozoology 17, 39–51 - PubMed
-
- Sherwin T and Gull K (1989) Visualization of Detyrosination along Single Microtubules Reveals Novel Mechanisms of Assembly during Cytoskeletal Duplication in Trypanosomes. Cell 57, 211–221 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

