Estrogen Down-regulator Fulvestrant Potentiates Antitumor Activity of Fluoropyrimidine in Estrogen-responsive MCF-7 Human Breast Cancer Cells
- PMID: 31471390
- PMCID: PMC6754987
- DOI: 10.21873/invivo.11622
Estrogen Down-regulator Fulvestrant Potentiates Antitumor Activity of Fluoropyrimidine in Estrogen-responsive MCF-7 Human Breast Cancer Cells
Abstract
Background: Endocrine therapy is clinically administered in hormone-responsive breast cancer. Combinations of fluoropyrimidine S-1 and an aromatase inhibitor or anti-estrogen are considered beneficial in Japan. Herein we assessed new combinations of S-1 and fulvestrant.
Patients and methods: Cytotoxicity of fulvestrant and 5-fluorouracil (5-FU) was assessed in hormone-responsive (MCF-7) and non-responsive (MDA-MB-231) breast cancer cell cultures. Fulvestrant and S-1 were evaluated for antitumor activity in mice and their effects on estrogen receptor (ER)-α and progesterone receptor (PgR) levels in MCF-7 xenografts using immunohistochemical methods.
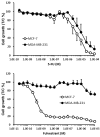
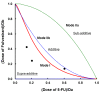
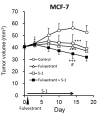
Results: Fulvestrant inhibited growth of MCF-7, but not of MDA-MB-231 xenografts. Combinations of 5-FU and fulvestrant were superior to monotherapy in vitro. In vivo antitumor activity of S-1/fulvestrant combination therapy was significantly (p<0.05) enhanced compared to that of both monotherapies. Fulvestrant partially down-regulated expression of ERα and PgR, but in combination with S-1, it almost completely blocked their expression.
Conclusion: Chemo-endocrine combination therapy using S-1 and fulvestrant is beneficial in estrogen-responsive breast cancer.
Keywords: Breast cancer; S-1; chemo-endocrine combination therapy; chemotherapy; endocrine therapy; estrogen receptor; fulvestrant; progesterone receptor.
Copyright© 2019, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
MN, HS, and TT are employees of Taiho Pharmaceutical Co., Ltd. SN has been an adviser for and received the honoraria and the research funding related to this study from Taiho Pharmaceutical Co., Ltd. (Tokyo, Japan); SN has also been an adviser for and received the honoraria and the research funding not related to this study from AstraZeneca (Cambridge, UK). This study was funded by Taiho Pharmaceutical Co., Ltd. (Tokyo, Japan). The Authors have no conflict of interest to declare regarding this study.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2018;68:7–30. PMID: 29313949. DOI: 10.3322/ caac.21442. - PubMed
-
- Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, Fallowfield L, Fowble B, Ingle JN, Jahanzeb M, Johnston SR, Korde LA, Khatcheressian JL, Mehta RS, Muss HB, Burstein HJ. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol. 2016;34:3069–3103. PMID: 27217461. DOI: 10.1200/JCO.2016.67.1487. - PubMed
-
- Vital Statistics Japan (Ministry of Health, Labour and Welfare). Available at: https://ganjoho.jp/reg_stat/statistics/dl/index.html.
-
- Sonoo H, Kurebayashi J, Iino Y, Inaji H, Watanabe T, Toi M, Kobayashi S, Sato B, Yoshimoto M. Current status and controversial issues concerning endocrine therapy for patients with recurrent breast cancer in Japan. Breast Cancer. 1999;6:344–355. PMID: 11091741. - PubMed
-
- Loibl S, Turner NC, Ro J, Cristofanilli M, Iwata H, Im SA, Masuda N, Loi S, André F, Harbeck N, Verma S, Folkerd E, Puyana Theall K, Hoffman J, Zhang K, Bartlett CH, Dowsett M. Palbociclib combined with fulvestrant in premenopausal women with advanced breast cancer and prior progression on endocrine therapy: PALOMA-3 results. Oncologist. 2017;22:1028–1038. PMID: 28652278. DOI: 10.1634/theoncologist. 2017-0072. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
