Outcome reporting from clinical trials of non-valvular atrial fibrillation treated with traditional Chinese medicine or Western medicine: a systematic review
- PMID: 31471437
- PMCID: PMC6720335
- DOI: 10.1136/bmjopen-2018-028803
Outcome reporting from clinical trials of non-valvular atrial fibrillation treated with traditional Chinese medicine or Western medicine: a systematic review
Abstract
Objectives: To examine variation in outcomes, outcome measurement instruments (OMIs) and measurement times in clinical trials of non-valvular atrial fibrillation (NVAF) and to identify outcomes for prioritisation in developing a core outcome set (COS) in this field.
Design: This study was a systematic review.
Data sources: Clinical trials published between January 2015 and March 2019 were obtained from PubMed, the Cochrane Library, Web of Science, Wanfang Database, the China National Knowledge Infrastructure and SinoMed.
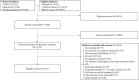
Eligibility criteria: Randomised controlled trials (RCTs) and observational studies were considered. Interventions included traditional Chinese medicine and Western medicine. The required treatment duration or follow-up time was ≥4 weeks. The required sample size was ≥30 and≥50 in each group in RCTs and observational studies, respectively. We excluded trials that aimed to investigate the outcome of complications of NVAF, to assess the mechanisms or pharmacokinetics, or for which full text could not be acquired.
Data extraction and synthesis: The general information and outcomes, OMIs and measurement times were extracted. The methodological and outcome reporting quality were assessed. The results were analysed by descriptive analysis.
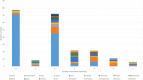
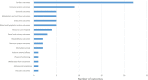
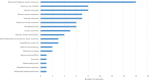
Results: A total of 218 articles were included from 25 255 articles. For clinical trials of antiarrhythmic therapy, 69 outcomes from 16 outcome domains were reported, and 28 (31.82%, 28/88) outcomes were reported only once; the most frequently reported outcome was ultrasonic cardiogram. Thirty-one outcomes (44.93%, 31/69) were provided definitions or OMIs; the outcome measurement times ranged from 1 to 20 with a median of 3. For clinical trials of anticoagulation therapy, 82 outcomes from 18 outcome domains were reported; 38 (29.23%, 38/130) outcomes were reported only once. The most frequently reported outcome was ischaemic stroke. Forty (48.78%, 40/82) outcomes were provided OMIs or definitions; and the outcome measurement times ranged from 1 to 27 with a median of 8.
Conclusion: Outcome reporting in NVAF is inconsistent. Thus, developing a COS that can be used in clinical trials is necessary.
Keywords: clinical trials, non-valvular atrial fibrillation; outcomes; systematic review.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



Similar articles
-
Development of a core outcome set (COS) and selecting outcome measurement instruments (OMIs) for non-valvular atrial fibrillation in traditional Chinese medicine clinical trials: study protocol.Trials. 2018 Oct 5;19(1):541. doi: 10.1186/s13063-018-2904-0. Trials. 2018. PMID: 30290840 Free PMC article.
-
Direct oral anticoagulants in the early phase of non valvular atrial fibrillation-related acute ischemic stroke: focus on real life studies.J Thromb Thrombolysis. 2019 Feb;47(2):292-300. doi: 10.1007/s11239-018-1775-2. J Thromb Thrombolysis. 2019. PMID: 30470967 Review.
-
Stroke Risk Status, Anticoagulation Treatment, and Quality-of-Life in Chinese Patients with Atrial Fibrillation: China Registry of Atrial Fibrillation (CRAF).Cardiovasc Ther. 2019 Mar 21;2019:7372129. doi: 10.1155/2019/7372129. eCollection 2019. Cardiovasc Ther. 2019. PMID: 31772614 Free PMC article.
-
A mixed methodology, non-interventional study to evaluate the use of direct oral anticoagulants in UK clinical practice for patients with a first stroke associated with non-valvular atrial fibrillation: study protocol.BMC Neurol. 2019 Nov 29;19(1):306. doi: 10.1186/s12883-019-1530-0. BMC Neurol. 2019. PMID: 31783738 Free PMC article.
-
A rapid evidence assessment of bleed-related healthcare resource utilization in publications reporting the use of direct oral anticoagulants for non-valvular atrial fibrillation.Curr Med Res Opin. 2019 Jan;35(1):127-139. doi: 10.1080/03007995.2018.1543184. Epub 2018 Dec 5. Curr Med Res Opin. 2019. PMID: 30380959 Review.
Cited by
-
Reporting Quality of Oral TCM Systematic Reviews Based on the PRISMA Harms Checklist from 2013 to 2020.Evid Based Complement Alternat Med. 2023 Jan 24;2023:4612036. doi: 10.1155/2023/4612036. eCollection 2023. Evid Based Complement Alternat Med. 2023. PMID: 36733845 Free PMC article.
-
Development of a core outcome set for myocardial infarction in clinical trials of traditional Chinese medicine: a study protocol.BMJ Open. 2019 Dec 3;9(12):e032256. doi: 10.1136/bmjopen-2019-032256. BMJ Open. 2019. PMID: 31796484 Free PMC article.
-
Developing an artificial intelligence method for screening hepatotoxic compounds in traditional Chinese medicine and Western medicine combination.Chin Med. 2022 May 17;17(1):58. doi: 10.1186/s13020-022-00617-4. Chin Med. 2022. PMID: 35581608 Free PMC article.
References
-
- January CT, Wann LS, Alpert JS, et al. . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American heart association Task force on practice guidelines and the heart rhythm Society. J Am Coll Cardiol 2014;64:e1–76. 10.1016/j.jacc.2014.03.022 - DOI - PubMed
-
- COMET Initiative Available: http://www.comet-initiative.org/ [Accessed 1 Apr 2019].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
