The efficacy and heterogeneity of antipsychotic response in schizophrenia: A meta-analysis
- PMID: 31471576
- PMCID: PMC7610422
- DOI: 10.1038/s41380-019-0502-5
The efficacy and heterogeneity of antipsychotic response in schizophrenia: A meta-analysis
Abstract
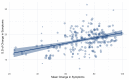
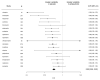
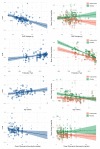
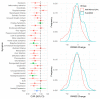
The response to antipsychotic treatment in schizophrenia appears to vary, and as such it has been proposed that different subtypes of schizophrenia exist, defined by treatment-response. This has not been formally examined using meta-analysis. Randomised controlled trials comparing placebo and antipsychotics in acute treatment of schizophrenia listed in PubMed, EMBASE and PsycINFO from inception until 30 November 2018 were examined. Relative variability of symptomatic improvement in antipsychotic-treated individuals compared to placebo-treated individuals was quantified using coefficient of variation ratio (CVR). Mean difference in symptom change was quantified using Hedges' g. In addition, individual patient data from two clinical trials was examined in terms of both the distribution of total symptom change, and the variability of individual symptoms and symptom factors. In total, 11,006 articles were identified. Sixty six met inclusion criteria, reporting on 17,202 patients. Compared with placebo, antipsychotic-treated patients demonstrated greater total symptom improvement (g = 0.47, p < 0.001) and reduced variability in symptomatic improvement for total (CVR = 0.86, p < 0.001), positive (CVR = 0.89, p < 0.001), and negative symptoms (CVR = 0.86, p = 0.001). Lower variability in antipsychotic-response relative to placebo was associated with studies published earlier (z = 3.98, p < 0.001), younger patients (z = 3.07, p = 0.002), higher dose treatments (z = -2.62, p = 0.009), and greater mean-difference in symptom-change (z = -5.70, p < 0.001). In the individual patient dataset (N = 522 patients), antipsychotic treated patients did not show significantly increased variability for any individual symptom, and there was no evidence of a bimodal distribution of response. Compared to placebo, antipsychotic treatment shows greater improvement and lower variability of change in total, positive and negative symptoms. This is contrary to the hypothesis that there is a subtype of antipsychotic non-responsive schizophrenia. Instead our findings, provide evidence for a relatively homogeneous effect of antipsychotic treatment in improving symptoms of schizophrenia.
Conflict of interest statement
RM, TP, AM,HP and LV declare no financial conflicts of interest. Dr. Mizuno has received manuscript fees or speaker’s honoraria from Sumitomo Dainippon Pharma and Yoshitomi Yakuhin, fellowship grants from Japan Society for the Promotion of Science, Astellas Foundation for Research on Metabolic Disorders, Japanese Society of Clinical Neuropsychopharmacology, and Mochida Memorial Foundation for Medical and Pharmaceutical Research, and consultant fees from Bracket within the past three years. ODH has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organised by Astra-Zeneca, Autifony, BMS, Eli Lilly, Heptares, Jansenn, Lundbeck, Lyden-Delta, Otsuka, Servier, Sunovion, Rand and Roche. Neither Dr Howes or his family have been employed by or have holdings/a financial stake in any biomedical company.
Figures

References
-
- Leucht S, Cipriani A, Spineli L, Mavridis D, Örey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;6736:1–12. - PubMed
-
- Agid O, Arenovich T, Sajeev G, Zipursky RB, Kapur S, Foussias G, et al. An algorithm-based approach to first-episode schizophrenia: Response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry. 2011;72:1439–1444. - PubMed
-
- Marques TR, Arenovich T, Agid O, Sajeev G, Muthén B, Chen L, et al. The different trajectories of antipsychotic response: antipsychotics versus placebo. Psychol Med. 2011;41:1481–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

