Apolipoprotein E-mediated regulation of selenoprotein P transportation via exosomes
- PMID: 31471680
- PMCID: PMC11104972
- DOI: 10.1007/s00018-019-03287-y
Apolipoprotein E-mediated regulation of selenoprotein P transportation via exosomes
Abstract
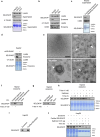
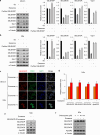
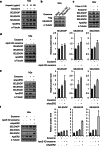
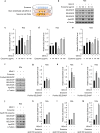
Selenoprotein P (SELENOP), secreted from the liver, functions as a selenium (Se) supplier to other tissues. In the brain, Se homeostasis is critical for physiological function. Previous studies have reported that SELENOP co-localizes with the apolipoprotein E receptor 2 (ApoER2) along the blood-brain barrier (BBB). However, the mechanism underlying SELENOP transportation from hepatocytes to neuronal cells remains unclear. Here, we found that SELENOP was secreted from hepatocytes as an exosomal component protected from plasma kallikrein-mediated cleavage. SELENOP was interacted with apolipoprotein E (ApoE) through heparin-binding sites of SELENOP, and the interaction regulated the secretion of exosomal SELENOP. Using in vitro BBB model of transwell cell culture, exosomal SELENOP was found to supply Se to brain endothelial cells and neuronal cells, which synthesized selenoproteins by a process regulated by ApoE and ApoER2. The regulatory role of ApoE in SELENOP transport was also observed in vivo using ApoE-/- mice. Exosomal SELENOP transport protected neuronal cells from amyloid β (Aβ)-induced cell death. Taken together, our results suggest a new delivery mechanism for Se to neuronal cells by exosomal SELENOP.
Keywords: Amyloid β; Apolipoprotein E; Exosome; Protein transport; Selenoprotein P.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Relationship between selenoprotein P and selenocysteine lyase: Insights into selenium metabolism.Free Radic Biol Med. 2018 Nov 1;127:182-189. doi: 10.1016/j.freeradbiomed.2018.03.037. Epub 2018 Mar 20. Free Radic Biol Med. 2018. PMID: 29567390 Free PMC article.
-
Uptake and Utilization of Selenium from Selenoprotein P.Biol Trace Elem Res. 2018 Jan;181(1):54-61. doi: 10.1007/s12011-017-1044-9. Epub 2017 May 9. Biol Trace Elem Res. 2018. PMID: 28488249 Free PMC article.
-
Role of selenoprotein P expression in the function of pancreatic β cells: Prevention of ferroptosis-like cell death and stress-induced nascent granule degradation.Free Radic Biol Med. 2022 Apr;183:89-103. doi: 10.1016/j.freeradbiomed.2022.03.009. Epub 2022 Mar 19. Free Radic Biol Med. 2022. PMID: 35318102
-
Selenoprotein P - Selenium transport protein, enzyme and biomarker of selenium status.Free Radic Biol Med. 2022 Oct;191:150-163. doi: 10.1016/j.freeradbiomed.2022.08.022. Epub 2022 Sep 5. Free Radic Biol Med. 2022. PMID: 36067902 Review.
-
Selenoprotein P and its potential role in Alzheimer's disease.Hormones (Athens). 2020 Mar;19(1):73-79. doi: 10.1007/s42000-019-00112-w. Epub 2019 Jun 27. Hormones (Athens). 2020. PMID: 31250406 Review.
Cited by
-
Excessive copper impairs intrahepatocyte trafficking and secretion of selenoprotein P.Nat Commun. 2023 Jun 13;14(1):3479. doi: 10.1038/s41467-023-39245-3. Nat Commun. 2023. PMID: 37311819 Free PMC article.
-
Bidirectional Control between Cholesterol Shuttle and Purine Signal at the Central Nervous System.Int J Mol Sci. 2022 Aug 4;23(15):8683. doi: 10.3390/ijms23158683. Int J Mol Sci. 2022. PMID: 35955821 Free PMC article. Review.
-
Selenium and Episodic Memory: The Moderating Role of Apolipoprotein E ε4.Nutrients. 2025 Feb 6;17(3):595. doi: 10.3390/nu17030595. Nutrients. 2025. PMID: 39940451 Free PMC article.
-
Selenium at the Neural Barriers: A Review.Front Neurosci. 2021 Feb 5;15:630016. doi: 10.3389/fnins.2021.630016. eCollection 2021. Front Neurosci. 2021. PMID: 33613188 Free PMC article. Review.
-
Copper metabolism in osteoarthritis and its relation to oxidative stress and ferroptosis in chondrocytes.Front Mol Biosci. 2024 Sep 11;11:1472492. doi: 10.3389/fmolb.2024.1472492. eCollection 2024. Front Mol Biosci. 2024. PMID: 39329090 Free PMC article. Review.
References
-
- Read R, Bellew T, Yang JG, Hill KE, Palmer IS, Burk RF. Selenium and amino acid composition of selenoprotein P, the major selenoprotein in rat serum. J Biol Chem. 1990;265(29):17899–17905. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

