Apolipoprotein E-mediated regulation of selenoprotein P transportation via exosomes
- PMID: 31471680
- PMCID: PMC11104972
- DOI: 10.1007/s00018-019-03287-y
Apolipoprotein E-mediated regulation of selenoprotein P transportation via exosomes
Abstract
Selenoprotein P (SELENOP), secreted from the liver, functions as a selenium (Se) supplier to other tissues. In the brain, Se homeostasis is critical for physiological function. Previous studies have reported that SELENOP co-localizes with the apolipoprotein E receptor 2 (ApoER2) along the blood-brain barrier (BBB). However, the mechanism underlying SELENOP transportation from hepatocytes to neuronal cells remains unclear. Here, we found that SELENOP was secreted from hepatocytes as an exosomal component protected from plasma kallikrein-mediated cleavage. SELENOP was interacted with apolipoprotein E (ApoE) through heparin-binding sites of SELENOP, and the interaction regulated the secretion of exosomal SELENOP. Using in vitro BBB model of transwell cell culture, exosomal SELENOP was found to supply Se to brain endothelial cells and neuronal cells, which synthesized selenoproteins by a process regulated by ApoE and ApoER2. The regulatory role of ApoE in SELENOP transport was also observed in vivo using ApoE-/- mice. Exosomal SELENOP transport protected neuronal cells from amyloid β (Aβ)-induced cell death. Taken together, our results suggest a new delivery mechanism for Se to neuronal cells by exosomal SELENOP.
Keywords: Amyloid β; Apolipoprotein E; Exosome; Protein transport; Selenoprotein P.
Conflict of interest statement
The authors declare no competing interests.
Figures
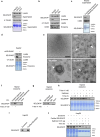

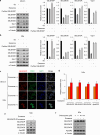
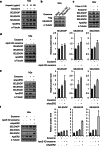
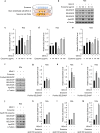


References
-
- Read R, Bellew T, Yang JG, Hill KE, Palmer IS, Burk RF. Selenium and amino acid composition of selenoprotein P, the major selenoprotein in rat serum. J Biol Chem. 1990;265(29):17899–17905. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

