Toward a robust tool for pharmacokinetic-based personalization of treatment with tacrolimus in solid organ transplantation: A model-based meta-analysis approach
- PMID: 31471970
- PMCID: PMC6955410
- DOI: 10.1111/bcp.14110
Toward a robust tool for pharmacokinetic-based personalization of treatment with tacrolimus in solid organ transplantation: A model-based meta-analysis approach
Abstract
Aims: The objective of this study is to develop a generic model for tacrolimus pharmacokinetics modelling using a meta-analysis approach, that could serve as a first step towards a prediction tool to inform pharmacokinetics-based optimal dosing of tacrolimus in different populations and indications.
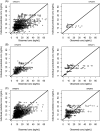
Methods: A systematic literature review was performed and a meta-model developed with NONMEM software using a top-down approach. Historical (previously published) data were used for model development and qualification. In-house individual rich and sparse tacrolimus blood concentration profiles from adult and paediatric kidney, liver, lung and heart transplant patients were used for model validation. Model validation was based on successful numerical convergence, adequate precision in parameter estimation, acceptable goodness of fit with respect to measured blood concentrations with no indication of bias, and acceptable performance of visual predictive checks. External validation was performed by fitting the model to independent data from 3 external cohorts and remaining previously published studies.
Results: A total of 76 models were found relevant for meta-model building from the literature and the related parameters recorded. The meta-model developed using patient level data was structurally a 2-compartment model with first-order absorption, absorption lag time and first-time varying elimination. Population values for clearance, intercompartmental clearance, central and peripheral volume were 22.5 L/h, 24.2 L/h, 246.2 L and 109.9 L, respectively. The absorption first-order rate and the lag time were fixed to 3.37/h and 0.33 hours, respectively. Transplanted organ and time after transplantation were found to influence drug apparent clearance whereas body weight influenced both the apparent volume of distribution and the apparent clearance. The model displayed good results as regards the internal and external validation.
Conclusion: A meta-model was successfully developed for tacrolimus in solid organ transplantation that can be used as a basis for the prediction of concentrations in different groups of patients, and eventually for effective dose individualization in different subgroups of the population.
Keywords: meta-analysis; pharmacodynamics; pharmacokinetics; pharmacometrics; population analysis; statistics; study design; tacrolimus.
© 2019 The British Pharmacological Society.
Figures





References
-
- Scalea JR, Levi ST, Ally W, Brayman KL. Tacrolimus for the prevention and treatment of rejection of solid organ transplants. Expert Rev Clin Immunol. 2016;12(3):333‐342. - PubMed
-
- Rath T. Tacrolimus in transplant rejection. Expert Opin Pharmacother. 2013;14(1):115‐122. - PubMed
-
- Holt CD. Overview of immunosuppressive therapy in solid organ transplantation. Anesthesiol Clin. 2017;35(3):365‐380. - PubMed
-
- Jahan A, Prabha R, Chaturvedi S, Mathew B, Fleming D, Agarwal I. Clinical efficacy and pharmacokinetics of tacrolimus in children with steroid‐resistant nephrotic syndrome. Pediatr Nephrol. 2015;30(11):1961‐1967. - PubMed
-
- Sikma MA, van Maarseveen EM, van de Graaf EA, et al. Pharmacokinetics and toxicity of tacrolimus early after heart and lung transplantation. Am J Transplant. 2015;15(9):2301‐2313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

