How do cardiologists select patients for dual antiplatelet therapy continuation beyond 1 year after a myocardial infarction? Insights from the EYESHOT Post-MI Study
- PMID: 31471975
- PMCID: PMC6837024
- DOI: 10.1002/clc.23262
How do cardiologists select patients for dual antiplatelet therapy continuation beyond 1 year after a myocardial infarction? Insights from the EYESHOT Post-MI Study
Abstract
Background: Current guidelines suggest to consider dual antiplatelet therapy (DAPT) continuation for longer than 12 months in selected patients with myocardial infarction (MI).
Hypothesis: We sought to assess the criteria used by cardiologists in daily practice to select patients with a history of MI eligible for DAPT continuation beyond 1 year.
Methods: We analyzed data from the EYESHOT Post-MI, a prospective, observational, nationwide study aimed to evaluate the management of patients presenting to cardiologists 1 to 3 years from the last MI event.
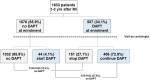
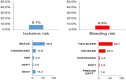
Results: Out of the 1633 post-MI patients enrolled in the study between March and December 2017, 557 (34.1%) were on DAPT at the time of enrolment, and 450 (27.6%) were prescribed DAPT after cardiologist assessment. At multivariate analyses, a percutaneous coronary intervention (PCI) with multiple stents and the presence of peripheral artery disease (PAD) resulted as independent predictors of DAPT continuation, while atrial fibrillation was the only independent predictor of DAPT interruption for patients both at the second and the third year from MI at enrolment and the time of discharge/end of the visit.
Conclusions: Risk scores recommended by current guidelines for guiding decisions on DAPT duration are underused and misused in clinical practice. A PCI with multiple stents and a history of PAD resulted as the clinical variables more frequently associated with DAPT continuation beyond 1 year from the index MI.
Keywords: clopidogrel; dual antiplatelet therapy; percutaneous coronary intervention; post-MI; secondary prevention; ticagrelor.
© 2019 The Authors. Clinical Cardiology published by Wiley Periodicals, Inc.
Conflict of interest statement
L.D.L. reports personal fees from Astra Zeneca and Daiichi Sankyo outside the submitted work. All other authors have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Figures

References
-
- Valgimigli M, Bueno H, Byrne RA, et al. ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J 2018;39:213–260. - PubMed
-
- Levine GN, Bates ER, Bittl JA, et al. ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and Management of Patients with Stable Ischemic Heart Disease, 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134:e123‐55. - PubMed
-
- Bonaca MP, Bhatt DL, Cohen M, et al. Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791‐1800. - PubMed
-
- Bavishi C, Trivedi V, Singh M, Katz E, Messerli FH, Bangalore S. Duration of dual antiplatelet therapy in patients with an acute coronary syndrome undergoing percutaneous coronary intervention. Am J Med. 2017;130:1325.e1‐1325.e12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

