Lessons learned from quantitative fundus autofluorescence
- PMID: 31472235
- PMCID: PMC7561015
- DOI: 10.1016/j.preteyeres.2019.100774
Lessons learned from quantitative fundus autofluorescence
Abstract
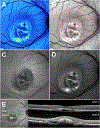
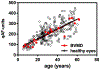
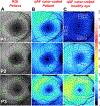
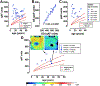
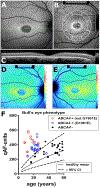
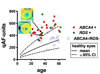
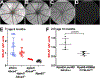
Quantitative fundus autofluorescence (qAF) is an approach that is built on a confocal scanning laser platform and used to measure the intensity of the inherent autofluorescence of retina elicited by short-wavelength (488 nm) excitation. Being non-invasive, qAF does not interrupt tissue architecture, thus allowing for structural correlations. The spectral features, cellular origin and topographic distribution of the natural autofluorescence of the fundus indicate that it is emitted from retinaldehyde-adducts that form in photoreceptor cells and accumulate, under most conditions, in retinal pigment epithelial cells. The distributions and intensities of fundus autofluorescence deviate from normal in many retinal disorders and it is widely recognized that these changing patterns can aid in the diagnosis and monitoring of retinal disease. The standardized protocol employed by qAF involves the normalization of fundus grey levels to a fluorescent reference installed in the imaging instrument. Together with corrections for magnification and anterior media absorption, this approach facilitates comparisons with serial images and images acquired within groups of patients. Here we provide a comprehensive summary of the principles and practice of qAF and we highlight recent efforts to elucidate retinal disease processes by combining qAF with multi-modal imaging.
Keywords: ABCA4; Acute zonal occult outer retinopathy; Age-related macular degeneration; Confocal scanning laser ophthalmoscopy; Fundus; Fundus autofluorescence; Quantitative fundus autofluorescence; Recessive stargardt disease; Retina; Retinitis pigmentosa.
Copyright © 2019. Published by Elsevier Ltd.
Figures





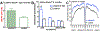
References
-
- Aizawa S, Mitamura Y, Hagiwara A, Sugawara T, Yamamoto S, 2010. Changes of fundus autofluorescence, photoreceptor inner and outer segment junction line, and visual function in patients with retinitis pigmentosa. Clin. Exp. Ophthalmol 38, 597–604. - PubMed
-
- ANSI, 2014. American National Standard for Safe Use of Lasers. (ANSI Z136.1). Laser Institute of America, Orlando, FL.
-
- Bakall B, Radu RA, Stanton JB, Burke JM, McKay BS, Wadelius C, Mullins RF, Stone EM, Travis GH, Marmorstein AD, 2007. Enhanced accumulation of A2E in individuals homozygous or heterozygous for mutations in BEST1 (VMD2). Exp. Eye Res 85, 34–43. - PubMed
-
- Ben-Shabat S, Itagaki Y, Jockusch S, Sparrow JR, Turro NJ, Nakanishi K, 2002. Formation of a nona-oxirane from A2E, a lipofuscin fluorophore related to macular degeneration, and evidence of singlet oxygen involvement. Angew. Chem. Int. Ed 41, 814–817. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

