Synthesis of a long acting nanoformulated emtricitabine ProTide
- PMID: 31472458
- PMCID: PMC6945494
- DOI: 10.1016/j.biomaterials.2019.119441
Synthesis of a long acting nanoformulated emtricitabine ProTide
Abstract
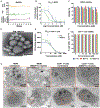
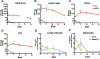
While antiretroviral therapy (ART) has revolutionized treatment and prevention of human immunodeficiency virus type one (HIV-1) infection, regimen adherence, viral mutations, drug toxicities and access stigma and fatigue are treatment limitations. These have led to new opportunities for the development of long acting (LA) ART including implantable devices and chemical drug modifications. Herein, medicinal and formulation chemistry were used to develop LA prodrug nanoformulations of emtricitabine (FTC). A potent lipophilic FTC phosphoramidate prodrug (M2FTC) was synthesized then encapsulated into a poloxamer surfactant (NM2FTC). These modifications extended the biology, apparent drug half-life and antiretroviral activities of the formulations. NM2FTC demonstrated a >30-fold increase in macrophage and CD4+ T cell drug uptake with efficient conversion to triphosphates (FTC-TP). Intracellular FTC-TP protected macrophages against an HIV-1 challenge for 30 days. A single intramuscular injection of NM2FTC, at 45 mg/kg native drug equivalents, into Sprague Dawley rats resulted in sustained prodrug levels in blood, liver, spleen and lymph nodes and FTC-TP in lymph node and spleen cells at one month. In contrast, native FTC-TPs was present for one day. These results are an advance in the transformation of FTC into a LA agent.
Keywords: Emtricitabine; Formulation; Long-acting slow effective release anti-retroviral therapy (LASER) ART; Prodrug.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures



References
-
- Andrews CD, Heneine W, Cabotegravir long-acting for HIV-1 prevention, Curr Opin HIV AIDS 10(4) (2015) 258–63. - PubMed
-
- Montgomery ET, van der Straten A, Chitukuta M, Reddy K, Woeber K, Atujuna M, Bekker LG, Etima J, Nakyanzi T, Mayo AJ, Katz A, Laborde N, Grossman CI, Soto-Torres L, Palanee-Phillips T, Baeten JM, Study M-A, Acceptability and use of a dapivirine vaginal ring in a phase III trial, AIDS 31(8) (2017) 1159–1167. - PMC - PubMed
-
- Benitez-Gutierrez L, Soriano V, Requena S, Arias A, Barreiro P, de Mendoza C, Treatment and prevention of HIV infection with long-acting antiretrovirals, Expert Rev Clin Pharmacol 11(5) (2018) 507–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS036126/NS/NINDS NIH HHS/United States
- R01 AI145542/AI/NIAID NIH HHS/United States
- P01 DA028555/DA/NIDA NIH HHS/United States
- P30 MH062261/MH/NIMH NIH HHS/United States
- P01 NS031492/NS/NINDS NIH HHS/United States
- T32 NS105594/NS/NINDS NIH HHS/United States
- R56 AI138613/AI/NIAID NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- R01 AG043540/AG/NIA NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
- R01 MH115860/MH/NIMH NIH HHS/United States
- P01 NS043985/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

