Acoustic diffusion constant of cortical bone: Numerical simulation study of the effect of pore size and pore density on multiple scattering
- PMID: 31472561
- PMCID: PMC6687498
- DOI: 10.1121/1.5121010
Acoustic diffusion constant of cortical bone: Numerical simulation study of the effect of pore size and pore density on multiple scattering
Abstract
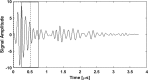
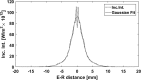
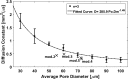
While osteoporosis assessment has long focused on the characterization of trabecular bone, the cortical bone micro-structure also provides relevant information on bone strength. This numerical study takes advantage of ultrasound multiple scattering in cortical bone to investigate the effect of pore size and pore density on the acoustic diffusion constant. Finite-difference time-domain simulations were conducted in cortical microstructures that were derived from acoustic microscopy images of human proximal femur cross sections and modified by controlling the density (Ct.Po.Dn) ∈[5-25] pore/mm2 and size (Ct.Po.Dm) ∈[30-100] μm of the pores. Gaussian pulses were transmitted through the medium and the backscattered signals were recorded to obtain the backscattered intensity. The incoherent contribution of the backscattered intensity was extracted to give access to the diffusion constant D. At 8 MHz, significant differences in the diffusion constant were observed in media with different porous micro-architectures. The diffusion constant was monotonously influenced by either pore diameter or pore density. An increase in pore size and pore density resulted in a decrease in the diffusion constant (D =285.9Ct.Po.Dm-1.49, R2=0.989 , p=4.96×10-5,RMSE=0.06; D=6.91Ct.Po.Dn-1.01, R2=0.94, p=2.8×10-3 , RMSE=0.09), suggesting the potential of the proposed technique for the characterization of the cortical microarchitecture.
Figures







Similar articles
-
Effect of porosity, tissue density, and mechanical properties on radial sound speed in human cortical bone.Med Phys. 2016 May;43(5):2030. doi: 10.1118/1.4942808. Med Phys. 2016. PMID: 27147315
-
Estimation of Cortical Bone Microstructure From Ultrasound Backscatter.IEEE Trans Ultrason Ferroelectr Freq Control. 2021 Apr;68(4):1081-1095. doi: 10.1109/TUFFC.2020.3033050. Epub 2021 Mar 26. IEEE Trans Ultrason Ferroelectr Freq Control. 2021. PMID: 33104498
-
The respective and dependent effects of scattering and bone matrix absorption on ultrasound attenuation in cortical bone.Phys Med Biol. 2024 May 20;69(11). doi: 10.1088/1361-6560/ad3fff. Phys Med Biol. 2024. PMID: 38631364
-
BMD-based assessment of local porosity in human femoral cortical bone.Bone. 2018 Sep;114:50-61. doi: 10.1016/j.bone.2018.05.028. Epub 2018 May 31. Bone. 2018. PMID: 29860154
-
The Influence of Cortical Porosity on the Strength of Bone During Growth and Advancing Age.Curr Osteoporos Rep. 2018 Oct;16(5):561-572. doi: 10.1007/s11914-018-0478-0. Curr Osteoporos Rep. 2018. PMID: 30187285 Review.
Cited by
-
Artificial neural network to estimate micro-architectural properties of cortical bone using ultrasonic attenuation: A 2-D numerical study.Comput Biol Med. 2019 Nov;114:103457. doi: 10.1016/j.compbiomed.2019.103457. Epub 2019 Sep 20. Comput Biol Med. 2019. PMID: 31600691 Free PMC article.
-
Ultrasound Scattering in Cortical Bone.Adv Exp Med Biol. 2022;1364:177-196. doi: 10.1007/978-3-030-91979-5_9. Adv Exp Med Biol. 2022. PMID: 35508876 Free PMC article.
-
Study of Ultrasonic Guided Wave Propagation in Bone Composite Structures for Revealing Osteoporosis Diagnostic Indicators.Materials (Basel). 2023 Sep 12;16(18):6179. doi: 10.3390/ma16186179. Materials (Basel). 2023. PMID: 37763457 Free PMC article.
-
In Vivo Assessment of Bone Quality Without X-rays.Curr Osteoporos Rep. 2024 Feb;22(1):56-68. doi: 10.1007/s11914-023-00856-w. Epub 2024 Jan 16. Curr Osteoporos Rep. 2024. PMID: 38227178 Free PMC article. Review.
-
Clinical Devices for Bone Assessment.Adv Exp Med Biol. 2022;1364:35-53. doi: 10.1007/978-3-030-91979-5_3. Adv Exp Med Biol. 2022. PMID: 35508870 Review.
References
-
- Aubry, A. , Derode, A. , and Padilla, F. (2008). “ Local measurements of the diffusion constant in multiple scattering media: Application to human trabecular bone imaging,” Appl. Phys. Lett. 92, 124101.10.1063/1.2901379 - DOI
-
- Bakalova, L. P. , Andreasen, C. M. , Thomsen, J. S. , Brüel, A. , Hauge, E.-M. , Kiil, B. J. , Delaisse, J.-M. , Andersen, T. L. , and Kersh, M. E. (2018). “ Intracortical bone mechanics are related to pore morphology and remodeling in human bone,” J. Bone Miner. Res. 33, 2177–2185.10.1002/jbmr.3561 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

