Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial
- PMID: 31472930
- PMCID: PMC6892281
- DOI: 10.1016/S0140-6736(19)31963-4
Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial
Abstract
Background: In women with late preterm pre-eclampsia, the optimal time to initiate delivery is unclear because limitation of maternal disease progression needs to be balanced against infant complications. The aim of this trial was to determine whether planned earlier initiation of delivery reduces maternal adverse outcomes without substantial worsening of neonatal or infant outcomes, compared with expectant management (usual care) in women with late preterm pre-eclampsia.
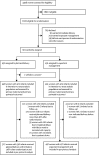
Methods: In this parallel-group, non-masked, multicentre, randomised controlled trial done in 46 maternity units across England and Wales, we compared planned delivery versus expectant management (usual care) with individual randomisation in women with late preterm pre-eclampsia from 34 to less than 37 weeks' gestation and a singleton or dichorionic diamniotic twin pregnancy. The co-primary maternal outcome was a composite of maternal morbidity or recorded systolic blood pressure of at least 160 mm Hg with a superiority hypothesis. The co-primary perinatal outcome was a composite of perinatal deaths or neonatal unit admission up to infant hospital discharge with a non-inferiority hypothesis (non-inferiority margin of 10% difference in incidence). Analyses were by intention to treat, together with a per-protocol analysis for the perinatal outcome. The trial was prospectively registered with the ISRCTN registry, ISRCTN01879376. The trial is closed to recruitment but follow-up is ongoing.
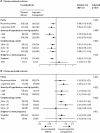
Findings: Between Sept 29, 2014, and Dec 10, 2018, 901 women were recruited. 450 women (448 women and 471 infants analysed) were allocated to planned delivery and 451 women (451 women and 475 infants analysed) to expectant management. The incidence of the co-primary maternal outcome was significantly lower in the planned delivery group (289 [65%] women) compared with the expectant management group (338 [75%] women; adjusted relative risk 0·86, 95% CI 0·79-0·94; p=0·0005). The incidence of the co-primary perinatal outcome by intention to treat was significantly higher in the planned delivery group (196 [42%] infants) compared with the expectant management group (159 [34%] infants; 1·26, 1·08-1·47; p=0·0034). The results from the per-protocol analysis were similar. There were nine serious adverse events in the planned delivery group and 12 in the expectant management group.
Interpretation: There is strong evidence to suggest that planned delivery reduces maternal morbidity and severe hypertension compared with expectant management, with more neonatal unit admissions related to prematurity but no indicators of greater neonatal morbidity. This trade-off should be discussed with women with late preterm pre-eclampsia to allow shared decision making on timing of delivery.
Funding: National Institute for Health Research Health Technology Assessment Programme.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Planned earlier delivery for late pre-eclampsia may be better for mothers.BMJ. 2020 Jan 15;368:l6779. doi: 10.1136/bmj.l6779. BMJ. 2020. PMID: 31941696
-
Managing late preterm pre-eclampsia.Lancet. 2020 Aug 1;396(10247):307-308. doi: 10.1016/S0140-6736(20)30622-X. Lancet. 2020. PMID: 32738943 No abstract available.
-
Managing late preterm pre-eclampsia.Lancet. 2020 Aug 1;396(10247):308. doi: 10.1016/S0140-6736(20)30623-1. Lancet. 2020. PMID: 32738946 No abstract available.
References
-
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. - PubMed
-
- ACOG ACOG Committee Opinion No 764: medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151–e155. - PubMed
-
- NICE . National Institute for Health and Care Excellence; London: 2010. Hypertension in pregnancy: the management of hypertensive disorders during pregnancy.
-
- Koopmans CM, Bijlenga D, Groen H. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks' gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374:979–988. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

