Inflammasomes: Threat-Assessment Organelles of the Innate Immune System
- PMID: 31473100
- PMCID: PMC6801093
- DOI: 10.1016/j.immuni.2019.08.005
Inflammasomes: Threat-Assessment Organelles of the Innate Immune System
Abstract
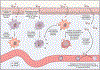
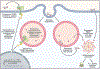
Inflammasomes are supramolecular organizing centers that operate to drive interleukin-1 (IL-1)-dependent inflammation. Depending on context, inflammatory caspases act upstream or downstream of inflammasome assembly, serving as the principal enzymes that control activities of these organelles. In this review, we discuss mechanisms of inflammasome assembly and signaling. We posit that upstream regulatory proteins, classically known as pattern-recognition receptors, operate to assess infectious and non-infectious threats to the host. Threat assessment is achieved through two general strategies: (1) direct binding of receptors to microbial or host-derived ligands or (2) indirect detection of changes in cellular homeostasis. Upon activation, these upstream regulatory factors seed the assembly of inflammasomes, leading to IL-1 family cytokine release from living (hyperactive) or dead (pyroptotic) cells. The molecular and physiological consequences of these distinct cell fate decisions are discussed.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
J.C.K. holds is a member of the scientific advisory board at IFM therapeutics and holds a patent on the therapeutic potential of hyperactivating stimuli (PCT/US2016/012994).
Figures


References
-
- Ayala JM, Yamin TT, Egger LA, Chin J, Kostura MJ, and Miller DK (1994). IL-1 beta-converting enzyme is present in monocytic cells as an inactive 45-kDa precursor. The Journal of Immunology 153, 2592–2599. - PubMed
-
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, et al. (2009). Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol 183, 787–791. - PMC - PubMed
-
- Blander JM, and Sander LE (2012). Beyond pattern recognition: five immune checkpoints for scaling the microbial threat. Nature Publishing Group 12, 215–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

