Salsalate, but not metformin or canagliflozin, slows kidney cyst growth in an adult-onset mouse model of polycystic kidney disease
- PMID: 31473186
- PMCID: PMC6796518
- DOI: 10.1016/j.ebiom.2019.08.041
Salsalate, but not metformin or canagliflozin, slows kidney cyst growth in an adult-onset mouse model of polycystic kidney disease
Abstract
Background: Multiple preclinical studies have highlighted AMP-activated protein kinase (AMPK) as a potential therapeutic target for autosomal dominant polycystic kidney disease (ADPKD). Both metformin and canagliflozin indirectly activate AMPK by inhibiting mitochondrial function, while salsalate is a direct AMPK activator. Metformin, canagliflozin and salsalate (a prodrug dimer of salicylate) are approved for clinical use with excellent safety profile. Although metformin treatment had been shown to attenuate experimental cystic kidney disease, there are concerns that therapeutic AMPK activation in human kidney might require a higher oral metformin dose than can be achieved clinically.
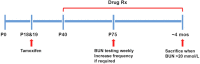
Methods: In this study, we tested metformin-based combination therapies for their additive (metformin plus canagliflozin) and synergistic (metformin plus salsalate) effects and each drug individually in an adult-onset conditional Pkd1 knock-out mouse model (n = 20 male/group) using dosages expected to yield clinically relevant drug levels.
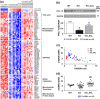
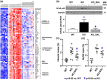
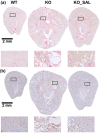
Findings: Compared to untreated mutant mice, treatment with salsalate or metformin plus salsalate improved kidney survival (i.e. blood urea nitrogen <20 mmol/L at the time of sacrifice) and reduced cystic kidney disease severity. However, the effects of metformin plus salsalate did not differ from salsalate alone; and neither metformin nor canagliflozin was effective. Protein expression and phosphorylation analyses indicated that salsalate treatment was associated with reduction in mTOR (mammalian target of rapamycin) activity and cellular proliferation in Pkd1 mutant mouse kidneys. Global gene expression analyses suggested that these effects were linked to restoration of mitochondrial function and suppression of inflammation and fibrosis.
Interpretation: Salsalate is a highly promising candidate for drug repurposing and clinical testing in ADPKD.
Keywords: AMPK; Canagliflozin; Metformin; Polycystic kidney disease; Salsalate.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Y.P. has served as consultant and received honoraria from Otsuka and Vertex Pharmaceutical. D.P. has served as consultant and received honorarium from Mironid. All other authors have nothing to disclose.
Figures


References
-
- Peters D.J., Sandkuijl L.A. Genetic heterogeneity of polycystic kidney disease in Europe. Contrib Nephrol. 1992;97:128–139. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

