Circulating tumour cells and hypercoagulability: a lethal relationship in metastatic breast cancer
- PMID: 31473984
- PMCID: PMC7188731
- DOI: 10.1007/s12094-019-02197-6
Circulating tumour cells and hypercoagulability: a lethal relationship in metastatic breast cancer
Abstract
Purpose: Circulating tumour cells (CTCs) are a marker of poor prognosis and are associated with increased risk of venous thromboembolism in metastatic breast cancer (MBC). We aimed to determine if the presence of CTCs and plasma markers of hypercoagulability [thrombin-antithrombin III (TAT), fibrinogen and D-dimer] are biomarkers of survival in MBC.
Methods/patients: In a prospective study of MBC patients, CTC (CellSearch®) enumeration and plasma TAT, fibrinogen and D-dimer measured prior to commencement of treatment for disease progression were correlated to overall survival.
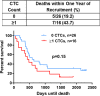
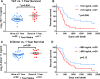
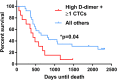
Results: At study completion, of 50 MBC patients recruited (median age 59 years, range 36-82), 40 patients had died (median survival 417 days, range 58-2141). CTCs (≥ 1/7.5 ml) were identified in 16 patients (median number of cells per 7.5 ml, 3 (range 1-31) and were associated with systemic hypercoagulability (medians TAT: 8.1 vs. 5.2 ng/ml, p = 0.03; fibrinogen: 4.3 vs. 3.1 g/l, p = 0.03; D-dimer: 1327 vs. 683 ng/ml, p = 0.0001). At 1 year, of 16 patients with ≥ 1 CTC, 7 had died (44%), compared to 5 of 26 (19%) patients in the no-CTC group. The presence of ≥ 1 CTC was associated with a trend for reduced overall survival (median 455 days vs. 614 days, p = 0.15). Plasma TAT inversely correlated with survival and was significantly higher in patients dying within 1 year (median 9.8 vs. 5.2 ng/ml, p = 0.004) whilst D-dimer showed a trend for reduced 1-year survival (median 1211 vs. 817 ng/ml, p = 0.06). MBC patients with combined high D-dimer (≥ 895 ng/ml) and CTC positivity (≥ 1/7.5 ml whole peripheral blood) had significantly reduced survival (p = 0.04).
Conclusions: The correlation between CTCs, hypercoagulability and reduced survival in MBC suggests the coagulation system supports tumour cell metastasis and is, therefore, a potential therapeutic target.
Keywords: CTC; Coagulation; D-Dimer; Fibrinogen; Survival; TAT.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Cronin-Fenton DP, Sondergaard F, Pedersen LA, Fryzek JP, Cetin K, Acquavella J, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997–2006. Br J Cancer. 2010;103(7):947–953. doi: 10.1038/sj.bjc.6605883. - DOI - PMC - PubMed
-
- Weiss RB, Tormey DC, Holland JF, Weinberg VE. Venous thrombosis during multimodal treatment of primary breast carcinoma. Cancer Treat Rep. 1981;65(7–8):677–679. - PubMed
-
- Levitan N, Dowlati A, Remick SC, Tahsildar HI, Sivinski LD, Beyth R, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore) 1999;78(5):285–291. doi: 10.1097/00005792-199909000-00001. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

