Redefining the Etiologic Landscape of Cerebellar Malformations
- PMID: 31474318
- PMCID: PMC6731369
- DOI: 10.1016/j.ajhg.2019.07.019
Redefining the Etiologic Landscape of Cerebellar Malformations
Abstract
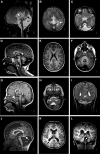
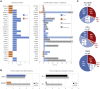
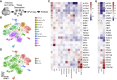
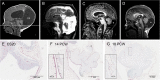
Cerebellar malformations are diverse congenital anomalies frequently associated with developmental disability. Although genetic and prenatal non-genetic causes have been described, no systematic analysis has been performed. Here, we present a large-exome sequencing study of Dandy-Walker malformation (DWM) and cerebellar hypoplasia (CBLH). We performed exome sequencing in 282 individuals from 100 families with DWM or CBLH, and we established a molecular diagnosis in 36 of 100 families, with a significantly higher yield for CBLH (51%) than for DWM (16%). The 41 variants impact 27 neurodevelopmental-disorder-associated genes, thus demonstrating that CBLH and DWM are often features of monogenic neurodevelopmental disorders. Though only seven monogenic causes (19%) were identified in more than one individual, neuroimaging review of 131 additional individuals confirmed cerebellar abnormalities in 23 of 27 genetic disorders (85%). Prenatal risk factors were frequently found among individuals without a genetic diagnosis (30 of 64 individuals [47%]). Single-cell RNA sequencing of prenatal human cerebellar tissue revealed gene enrichment in neuronal and vascular cell types; this suggests that defective vasculogenesis may disrupt cerebellar development. Further, de novo gain-of-function variants in PDGFRB, a tyrosine kinase receptor essential for vascular progenitor signaling, were associated with CBLH, and this discovery links genetic and non-genetic etiologies. Our results suggest that genetic defects impact specific cerebellar cell types and implicate abnormal vascular development as a mechanism for cerebellar malformations. We also confirmed a major contribution for non-genetic prenatal factors in individuals with cerebellar abnormalities, substantially influencing diagnostic evaluation and counseling regarding recurrence risk and prognosis.
Keywords: Dandy-Walker malformation; autism; cerebellar hypoplasia; cerebellum; epilepsy; exome; genes; heterotopia; intellectual disability; twins.
Copyright © 2019 American Society of Human Genetics. All rights reserved.
Conflict of interest statement
G.S., A.B.R., and C.R. have filed a patent related to the SPLiT-seq method. All other authors declare no competing interests.
Figures
References
-
- Pinchefsky E.F., Accogli A., Shevell M.I., Saint-Martin C., Srour M. Developmental outcomes in children with congenital cerebellar malformations. Dev. Med. Child Neurol. 2019;61:350–358. - PubMed
-
- Limperopoulos C., Robertson R.L., Sullivan N.R., Bassan H., du Plessis A.J. Cerebellar injury in term infants: clinical characteristics, magnetic resonance imaging findings, and outcome. Pediatr. Neurol. 2009;41:1–8. - PubMed
-
- Kontopoulos E.V., Quintero R.A., Salihu H.M., Bornick P.W., Allen M.H. Dandy-Walker syndrome and monochorionic twins: insight into a possible etiological mechanism. J. Matern. Fetal Neonatal Med. 2008;21:839–842. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

