Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence
- PMID: 31474332
- PMCID: PMC6891893
- DOI: 10.1016/S0140-6736(19)31709-X
Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence
Abstract
Background: Published findings on breast cancer risk associated with different types of menopausal hormone therapy (MHT) are inconsistent, with limited information on long-term effects. We bring together the epidemiological evidence, published and unpublished, on these associations, and review the relevant randomised evidence.
Methods: Principal analyses used individual participant data from all eligible prospective studies that had sought information on the type and timing of MHT use; the main analyses are of individuals with complete information on this. Studies were identified by searching many formal and informal sources regularly from Jan 1, 1992, to Jan 1, 2018. Current users were included up to 5 years (mean 1·4 years) after last-reported MHT use. Logistic regression yielded adjusted risk ratios (RRs) comparing particular groups of MHT users versus never users.
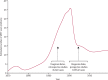
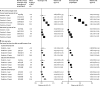
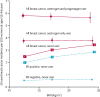
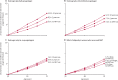
Findings: During prospective follow-up, 108 647 postmenopausal women developed breast cancer at mean age 65 years (SD 7); 55 575 (51%) had used MHT. Among women with complete information, mean MHT duration was 10 years (SD 6) in current users and 7 years (SD 6) in past users, and mean age was 50 years (SD 5) at menopause and 50 years (SD 6) at starting MHT. Every MHT type, except vaginal oestrogens, was associated with excess breast cancer risks, which increased steadily with duration of use and were greater for oestrogen-progestagen than oestrogen-only preparations. Among current users, these excess risks were definite even during years 1-4 (oestrogen-progestagen RR 1·60, 95% CI 1·52-1·69; oestrogen-only RR 1·17, 1·10-1·26), and were twice as great during years 5-14 (oestrogen-progestagen RR 2·08, 2·02-2·15; oestrogen-only RR 1·33, 1·28-1·37). The oestrogen-progestagen risks during years 5-14 were greater with daily than with less frequent progestagen use (RR 2·30, 2·21-2·40 vs 1·93, 1·84-2·01; heterogeneity p<0·0001). For a given preparation, the RRs during years 5-14 of current use were much greater for oestrogen-receptor-positive tumours than for oestrogen-receptor-negative tumours, were similar for women starting MHT at ages 40-44, 45-49, 50-54, and 55-59 years, and were attenuated by starting after age 60 years or by adiposity (with little risk from oestrogen-only MHT in women who were obese). After ceasing MHT, some excess risk persisted for more than 10 years; its magnitude depended on the duration of previous use, with little excess following less than 1 year of MHT use.
Interpretation: If these associations are largely causal, then for women of average weight in developed countries, 5 years of MHT, starting at age 50 years, would increase breast cancer incidence at ages 50-69 years by about one in every 50 users of oestrogen plus daily progestagen preparations; one in every 70 users of oestrogen plus intermittent progestagen preparations; and one in every 200 users of oestrogen-only preparations. The corresponding excesses from 10 years of MHT would be about twice as great.
Funding: Cancer Research UK and the Medical Research Council.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures



Comment in
-
Menopausal hormone therapy and 20-year breast cancer mortality.Lancet. 2019 Sep 28;394(10204):1139. doi: 10.1016/S0140-6736(19)32033-1. Epub 2019 Aug 29. Lancet. 2019. PMID: 31474331 No abstract available.
-
HRT and breast cancer risk.BMJ. 2019 Oct 11;367:l5928. doi: 10.1136/bmj.l5928. BMJ. 2019. PMID: 31604711 No abstract available.
-
Risk of Breast Cancer Rises with Duration of Menopausal Hormone Therapy.Am J Nurs. 2019 Dec;119(12):14. doi: 10.1097/01.NAJ.0000615724.31763.c7. Am J Nurs. 2019. PMID: 31764035
-
Hormone replacement therapy and the risk of breast cancer: How much should women worry about it?Post Reprod Health. 2019 Dec;25(4):175-178. doi: 10.1177/2053369119898586. Post Reprod Health. 2019. PMID: 31941376 No abstract available.
-
Menopausal hormone therapy and breast cancer risk: the cardiological point of view.J Cardiovasc Med (Hagerstown). 2020 Aug;21(8):538-539. doi: 10.2459/JCM.0000000000000969. J Cardiovasc Med (Hagerstown). 2020. PMID: 32332382 No abstract available.
-
Hormone Therapy, Breast Cancer Risk and the Collaborative Group on Hormonal Factors in Breast Cancer Article.Rev Bras Ginecol Obstet. 2020 May;42(5):233-234. doi: 10.1055/s-0040-1712941. Epub 2020 May 29. Rev Bras Ginecol Obstet. 2020. PMID: 32483802 Free PMC article. No abstract available.
References
-
- Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52 705 women with breast cancer and 108 411 women without breast cancer. Lancet. 1997;350:1047–1059. - PubMed
-
- WHO International Agency for Research on Cancer CI5plus. Cancer Incidence in Five Continents Time Trends. Graphs: time trends by age. ci5.iarc.fr/CI5plus/Pages/graph2_sel.aspx
-
- European Medicines Agency Guidelines on clinical investigation of medicinal products for hormone replacement therapy of oestrogen deficiency symptoms in postmenopausal women. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/...
-
- US Food and Drug Administration Menopause. www.fda.gov/consumers/womens-health-topics/menopause
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

