A Cold-Sensing Receptor Encoded by a Glutamate Receptor Gene
- PMID: 31474366
- PMCID: PMC6743979
- DOI: 10.1016/j.cell.2019.07.034
A Cold-Sensing Receptor Encoded by a Glutamate Receptor Gene
Abstract
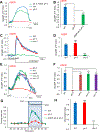
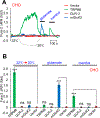
In search of the molecular identities of cold-sensing receptors, we carried out an unbiased genetic screen for cold-sensing mutants in C. elegans and isolated a mutant allele of glr-3 gene that encodes a kainate-type glutamate receptor. While glutamate receptors are best known to transmit chemical synaptic signals in the CNS, we show that GLR-3 senses cold in the peripheral sensory neuron ASER to trigger cold-avoidance behavior. GLR-3 transmits cold signals via G protein signaling independently of its glutamate-gated channel function, suggesting GLR-3 as a metabotropic cold receptor. The vertebrate GLR-3 homolog GluK2 from zebrafish, mouse, and human can all function as a cold receptor in heterologous systems. Mouse DRG sensory neurons express GluK2, and GluK2 knockdown in these neurons suppresses their sensitivity to cold but not cool temperatures. Our study identifies an evolutionarily conserved cold receptor, revealing that a central chemical receptor unexpectedly functions as a thermal receptor in the periphery.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures





References
-
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, and Julius D (2007). The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208. - PubMed
-
- Brockie PJ, Madsen DM, Zheng Y, Mellem J, and Maricq AV (2001). Differential expression of glutamate receptor subunits in the nervous system of Caenorhabditis elegans and their regulation by the homeodomain protein UNC-42. The Journal of neuroscience : the official journal of the Society for Neuroscience 21, 1510–1522. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

