ω-Hydroxy isoprenoid bisphosphonates as linkable GGDPS inhibitors
- PMID: 31474482
- PMCID: PMC6936606
- DOI: 10.1016/j.bmcl.2019.126633
ω-Hydroxy isoprenoid bisphosphonates as linkable GGDPS inhibitors
Abstract
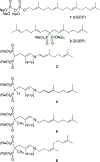
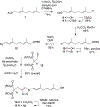
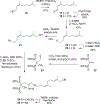
The enzyme geranylgeranyl diphosphate synthase (GGDPS) is a potential therapeutic target for multiple myeloma. Malignant plasma cells produce and secrete large amounts of monoclonal protein, and inhibition of GGDPS results in disruption of protein geranylgeranylation which in turn impairs intracellular protein trafficking. Our previous work has demonstrated that some isoprenoid triazole bisphosphonates are potent and selective inhibitors of GGDPS. To explore the possibility of selective delivery of such compounds to plasma cells, new analogues with an ω-hydroxy group have been synthesized and examined for their enzymatic and cellular activity. These studies demonstrate that incorporation of the ω-hydroxy group minimally impairs GGDPS inhibitory activity. Furthermore conjugation of one of the novel ω-hydroxy GGDPS inhibitors to hyaluronic acid resulted in enhanced cellular activity. These results will allow future studies to focus on the in vivo biodistribution of HA-conjugated GGDPS inhibitors.
Keywords: Bisphosphonate; GGDP synthase; Inhibition; Isoprenoid biosynthesis; Triazole.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures
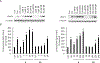


References
-
- Davis EM; Croteau R, Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes In Biosynthesis: Aromatic Polyketides, Isoprenoids, Alkaloids, Leeper FJ; Vederas JC, Eds. 2000; Vol. 209, pp 53–95.
-
- Wiemer AJ; Wiemer DF; Hohl RJ, Geranylgeranyl Diphosphate Synthase: An Emerging Therapeutic Target. Clin. Pharmacol. Ther. 2011, 90 (6), 804–812. - PubMed
-
- Chou R; Dana T; Blazina I; Daeges M; Jeanne TL, Statins for Prevention of Cardiovascular Disease in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. Jama 2016, 316 (19), 2008–2024. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Medical

