Introducing rotavirus vaccine in the Universal Immunization Programme in India: From evidence to policy to implementation
- PMID: 31474519
- PMCID: PMC6996154
- DOI: 10.1016/j.vaccine.2019.07.104
Introducing rotavirus vaccine in the Universal Immunization Programme in India: From evidence to policy to implementation
Abstract
Background: In 2016, India became one of the first countries in Asia to introduce an indigenously manufactured rotavirus vaccine. However, any new vaccine introduction needs to be meticulously planned to allow for strengthening of the existing immunization systems instead of burdening them.
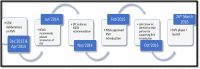
Methods: The process of rotavirus vaccine introduction in India started with the establishment of National Rotavirus Surveillance Network in 2005 which generated relevant evidence to inform policy level decisions to introduce the vaccine. The preparatory activities started with assessment of health systems and closing any gaps. This was followed by development of vaccine specific training packages and cascade training for programme managers and health workers. The introduction was complemented with strong communications systems and media involvement to allow for good acceptability of the vaccine on the ground. Each step of introduction was led by the government and technically supported by development partners.
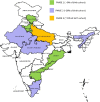
Results: India introduced rotavirus vaccine in a phased wise manner. In the first two phases the vaccine has been introduced in nine states of the country accounting for nearly 35% of the annual birth cohort of the country. From March 2016 to November 2017, approximately 13,260,000 rotavirus vaccine doses were administered in the country. The vaccine was well accepted by both the health workers and parents/caregivers.
Conclusion: Rotavirus vaccine introduction in India is an excellent example of how government stewardship with well-defined roles for development partners can allow a new vaccine introduction to be used as a system strengthening activity.
Keywords: Diarrhea; Immunization; Rotavirus vaccine.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Registrar General, India. Sample Registration System. New Delhi. December 2011; 46 (1). ISSN 0971-3549, <http://censusindia.gov.in/vital_statistics/SRS_Bulletins/SRS_Bulletin_De... [assessed 22 May 2018].
-
- Registrar General, India. Sample Registration System. New Delhi. May 2019; 52 (1). ISSN 0971-3549, <http://censusindia.gov.in/vital_statistics/SRS_Bulletins/SRS_Bulletin-Ra...> [assessed 1st June 2019].
-
- UNICEF. UNICEF Data: Monitoring the situation of children and women; 2017. <https://data.unicef.org/country/ind/> [assessed 22nd May 2018].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical